Triacontanol as a Growth Stimulant - A Report of Two Experiments
By D.L Hinerman, M.D. and S.L. Kunkel, Ph.D., Ann Arbor, Michigan
Introduction
For many years beneficial effects of alfalfa on plants, animals and humans have been suspected. In the mid-seventies intense interest was generated by Stanley K. Ries et. al. when they observed significant increases in growth and productivity of plants which were in close proximity to alfalfa hay (Medicago sativa L). Further investigation led to the isolation of the active principle in alfalfa, 1-triacontanol. Alfalfa and triacontanol in small quantities not only stimulated growth and productivity of plants, but did so in the dark independently of photosynthesis.
1,3,4,5,6,7,8
1-Triacontanol (CH
3
CH
28
CH
2
OH) is a ubiquitous long chain alcohol found incorporated in the surface of many plants and is a major component of beeswax.
Experiment #1 Growth of Animal Cells
The effects of triacontanol on growth and productivity of plants is so striking that questions might be raised about similar effects on mammalian cells. With the great fears about environmental cancer-producing growth, it was considered wise to test triacontanol for growth promoting activity in mammalian cells.
Growing cells having active nuclear and cellular division with increase in DNA will incorporate thymidine, including radioactive
3
H-thymidine. The process of nuclear and cellular division can be stimulated in cells by substances such as phytohemagglutinin (PHA). Cells treated with PHA then can be compared with similar cells treated with or without triacontanol. Quantitative counts of the amounts of radioactivity in each of the three groups of cells will reveal the relative amount of growth stimulation or DNA synthesis as shown by
3
H-thymidine incorporation.
2
This procedure was performed using cells derived from the spleens of CBA-J female mice from Jackson Laboratories in Bar Harbor, Maine. These spleens were removed by aseptic technique, were teased apart and gently pressed through a fine stainless steel mesh into a culture medium. The cells were 90-95% viable. Spleen cells were diluted to a concentration of 5x10
6
/ml in culture medium with 10% fetal calf serum containing penicillin and streptomycin. To one series of cultures containing the above cells were added 0.001 to 10 g/ml triacontanol solution, to another series 0.001 to 10 g/ml of phytohemagglutinin, the known stimulant and to a third series nothing was added. All cultures were incubated for 60 hours, then
3
H-thymidine was added to each of the cultures and incubated for another 12 hours.
Results
3
H-thymidine incorporation
As shown in Figure I, there was no statistically significant increase in any of the triacontanol treated cultures above the non-treated cells. No increase was observed over a wide range of triacontanol concentrations 1 g/ml to 0.001 g/ml. On the other hand the PHA treated cultures responded in a normal fashion with a peak response at approximately 0.1 g PHA/ml.
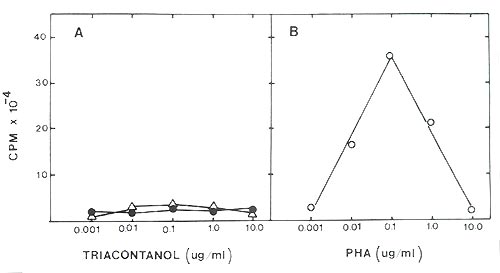
|
|
Fig. I. Incorporation of
3
H-thymidine by mouse spleen cell cultures.
A) Triacontanol (ΔΔΔ) over a broad concentration range did not increase 3 HdT incorporation over controls (●●●). B) PHA (phytohemagglutinin) (ooo) demonstrated a characteristic dose response curve with suppression of 3 HdT incorporation occurring at high doses and a maximum response at 0.1 g PHA/ml. |
Experiment #2 Growth of Plants
Although some scientists and plant growers have seen remarkable increases in growth and production of plants from alfalfa and triacontanol, subsequent field tests by Ries and by other scientists from the U.S.D.A., universities and chemical companies have proved inconsistent and disappointing.
5
Ries attributes most of the problems to the methods of formulation of triacontanol in that the long chain alcohol is very insoluble in water and in most other solvents. Ries and others have used combinations of solvents, detergents and surfactants in efforts to keep triacontanol in solution. The authors had a batch of triacontanol that had been opened for several months and this sample defied all attempts to get it in solution. A.J. Welebir is reported to have found additional requirements such as a solution at a pH of 8 or higher and the presence of metal ions such as calcium in the triacontanol solution. Using a solution of triacontanol with the above additions, Welebir has produced even more amazing growth and production than others have achieved and has done it consistently.
5
Materials for experiment #2 consisted of eight first-year rooted cuttings (clones) of genetically identical plants of W. Delp's hybrid rhododendron (R. pink carolinianum x R. scintillans) #1. The cuttings were about the same size, consisting of one or two stems, small leaves and fairly uniform small root systems. They were placed in a rooting bed in a cold greenhouse (minimum temperature was 40°F.) The soil mixture was equal parts of peat and perlite. Bottom heat of 80°F was present. One mg of 1-triacontanol was dissolved in one ml of chloroform, carefully diluted with de-ionized water to make a 0.1 mg/liter solution. (Ries had found that aqueous solutions containing small amounts of chloroform had no effect on plants.) The pH of the solution was 5.3. The pH of the rooting mixture was 5.5. No calcium ions were present except in buffers found in the well-water (pH 7) with which the plants were sprayed at frequent intervals.
The trial began in mid-January when plants were dormant. Six of the eight plants were painted thoroughly with the triacontanol solution and this was repeated in one week and again in mid-April. In May the rooted cuttings were transplanted to individual pots. In June only four of the 8 plants were sprayed and in August only 2 of the 8 plants were sprayed making 5 the maximum number of doses for two of the plants.
Figure II illustrates the difference in growth between the total controls and the plants receiving 5 treatments. Five different measurements of growth were taken in early October.
| Figure II Measurements of five different aspects of growth of rooted cuttings of a rhododendron hybrid, both untreated and treated with 0.1 mg triacontanol/liter. | |||
| TYPE OF MEASUREMENT | CONTROL PLANTS | PLANTS TREATED 5 TIMES | INCREASE |
| Total Weight (minus soil) | 30.2 g | 83.1 g | 275% |
| Diameter of trunk at base | 4.0 mm | 8.0mm | 100% |
| Number of branches | 4 | 10 | 150% |
| Number of leaves | 62 | 167 | 269% |
| Number of flower buds | 4 | 8 | 100% |
The first results of the experiment was an obvious premature break in dormancy within the first two weeks in the triacontanol treated plants (end of January), whereas dormancy did not end until early May in the control plants. After 2 months the growth in treated plants was double that of the untreated plants. At the end of the growing season the amount of growth roughly paralleled the number and duration of treatments. A fantastic amount of growth had occurred especially in those plants which had 4 and 5 treatments over most of the growing season. Those receiving 5 treatments had tertiary branching as compared to secondary branching in those with 3 and 4 treatments and primary branching in the remainder. The root system after 5 treatments appeared proportionately larger than other aspects of the plant. One of the most profound differences was that of a total weight of 2 times greater in maximally treated plants as compared to untreated plants. Of great interest also was the double number of blossom buds in maximally treated plants and the diameter of those buds was twice that of the untreated plants.

|
|
Figure III Photograph illustrating a control or untreated
plant on the left side compared with two triacontanol treated plants (center and right side) of identical rooted cuttings of (R. pink carolinianum x R. scintillans) #1. The plant in the center was treated five times through August; the plant on the right was treated four times through June. |
Discussion
Results of these two studies indicated that 1 -triacontanol can act as a powerful plant growth stimulant; yet this long chain lipid alcohol appears to have no stimulatory effect on mammalian DNA increase, since there was no increase in the uptake of
3
H-thymidine by mouse spleen cells. Of course the Environmental Protection Agency must approve triacontanol before it can be used commercially. At least we can use it in trials with the knowledge that it probably will not influence the growth of cells in man and animals.
Scientists have been mystified by the mechanism of action of triacontanol on plants. We have nothing to add to solve the mystery of why the addition of trace amounts of triacontanol to a plant which already had much greater amounts of this chemical will produce doubling or tripling of growth in some instances. Why or how does it increase flowering and productivity? How does triacontanol replace light, produce growth in the dark and break dormancy in plants? All of these are highly desirable qualities.
Crop production, like blossom and seed production, is dramatically increased and at trifling cost. Rooting of cuttings and seedling production is enhanced.
It is obvious that the proper use of 1-triacontanol and other newly discovered natural substances will revolutionize the plant and farming industries. The world-wide mass starvation of people, which has been predicted because of our destruction of farmland, may be delayed.
Nurserymen can produce larger, healthier and more mature plants in much less time and less expense than at present, also they will be able to force a greater variety of plants with more numerous and larger flowers than formerly possible. These changes do not appear to reduce hardiness in the plants. These are but a few examples of changes which will guarantee a revolution in the plant industry with the use of these new plant growth regulators.
References
1. Bittender, H.C.; Dilley, D.A.; Wert, V. and Ries, S.K.: Environmental Parameters Affecting Dark Response of Rice Seedlings (oryza sativa L) to Triacontanol. Plant Physiol. 61:851-854, 1978
2. Cave, M.D.: Incorporation of Tritium-labeled Thymidine and Lysine into Chromosomes of Cultured Human Leukocytes. J. Cell Biol. 29:209-220, 1966
3. Hangarter, R.; Ries, S.K. and Carlson, P.: Effect of Triacontanol on Plant Cell Cultures In Vitro. Plant Physiol. 61:855-857, 1978
4. Hinerman, D.L.: Stimulation of Growth by Alfalfa. Quarterly Bulletin. American Rhododendron Society 33:70-73, 1979
5. Maugh, T.H., II. New Chemicals Promise Larger Crops. Science 212:33-34, 3 April, 1981
6. Jones, J.; Wert, V. and Ries, S.K.: Specificity of 1-Triacontanol as a Plant Growth Stimulator and Inhibition of its Effects by other Long Chain Compounds. Planta 144:277-282, 1979
7. Ries, S.K.; Wert, V.F.; Sweeley, C.C. and Leavitt, R.A.: Triacontanol: A New Naturally Occurring Plant Growth Regulator. Science 195:1339-1341, 25 March, 1977
8. Ries, S.K.; Richman, T.L.; Wert, V.F.: Growth and Yield of Crops Treated with Triacontanol. J. Amer. Soc. Hort. Sci. 103(3):361-364, May 1978
Acknowledgments
We express thanks to Mrs. Robin Kunkel for preparing the chart, graph and photograph in a pleasing manner, Mrs. Marjorie Owens for secretarial assistance and Mr. Craig Biddie and Mr. Eddie Burks for the photographs.