A Vireya Azaleodendron In Flower
J.L. Rouse, E.G. Williams and R.B. Knox
The University of Melbourne
Parkville, Victoria, Australia
Introduction
The first flowering of a new rhododendron hybrid is always an exciting event and something to await with pleasure, even if it is only an F1 hybrid which can be expected to show characteristics intermediate between those of the parent species. When, however, the two parents are so well separated taxonomically and geographically that a successful cross has initially seemed most unlikely, the appearance of a hybrid flower bud generates intense excitement and speculation. Here, we describe the first flowering of a
Vireya
x azalea,
R. retusum
x
R. nudiflorum
, a hybrid that spans the lepidote-elepidote breeding barrier and which as far as we can ascertain, represents the first report of flowering of a genuine cross between a
Vireya
and a
non-Vireya.
The genus
Rhododendron
comprises two distinct groups, subgenus
Rhododendron
, the lepidotes, and the other seven subgenera, the elepidotes, which include subgenus
Hymenanthes
and the Azalea Complex. Attempts to produce hybrids between these two groups usually fail, though a number of successes have been reported (Martin, 1963; Heyting, 1970; Kehr, 1977), of which
R. griersonianum
x
R. dalhousiae
, 'Grierdal' is the first recorded and verified (Waterson, 1940) and probably the best known. Mostly the successful crosses have been azaleodendrons, with one parent in the Azalea Complex and the other in section
Rhododendron
(Kehr, 1977). Lepidote-elepidote hybrid seedlings have also been obtained with the scaley parent from section
Vireya
. Seedlings of
Vireya
hybrid x evergreen azalea were obtained by Messrs Veitch and Sons (Henslow, 1891), and seedlings of
Vireya
species x evergreen azalea were produced by Holttum (1941). All genuine
Vireya
x azalea seedlings lacked vigour and died well before reaching flowering size. One seedling which flowered and was described by Henslow, was almost certainly a
Vireya
x
Vireya
hybrid. More recently, as part of a program to investigate sexual compatibility barriers in
Rhododendron
(Williams et al., 1982 and 1985), we obtained viable seed from crosses with the female parent in section
Vireya
and the male parent in section
Pentanthera
, section
Tsutsusi
or section
Choniastrum
. The first of these hybrids to flower is
R. retusum
x
R. nudiflorum
.
|

|
|
|
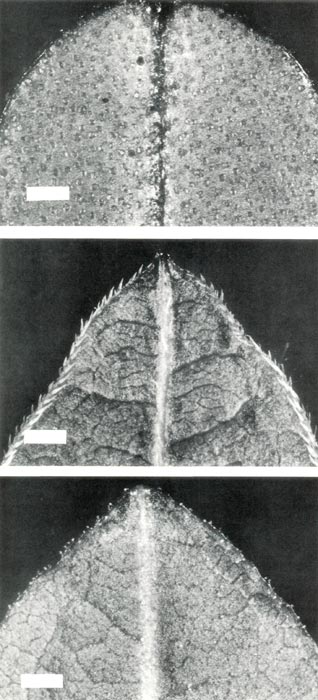
Figure 1: The lower surface at the apex
of young leaves from mature plants of (upper, a) R. retusum , showing scales; (center, b) R. nudiflorum , showing pointed hairs; (lower, c) R. retusum x R. nudiflorum , showing stalked glandular hairs. The bars are equivalent to a length of 1 mm. |
Figure 2: In flower (upper, a) the female
parent, R. retusum ; (center, b) the male parent, R. nudiflorum ; (lower, c) their hybrid, R. retusum x R. nudiflorum . |
|
|
|
|
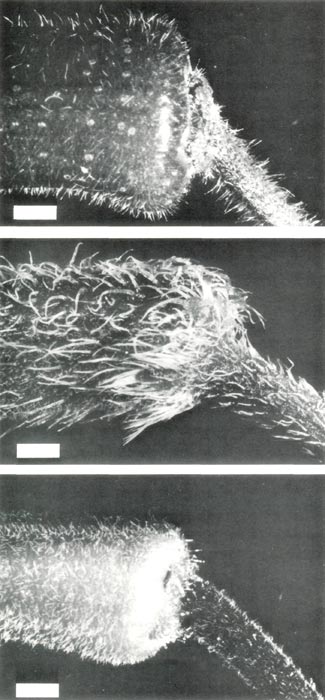
Figure 3: The base of the corolla and
pedical of (upper, a) R. retusum , showing short pointed hairs and scales; (center, b) R. nudiflorum , showing short and long pointed hairs; (lower, c) R. retusum x R. nudiflorum , showing short pointed hairs and a few stalked glandular hairs. The bars are equivalent to a length of 1 mm. |
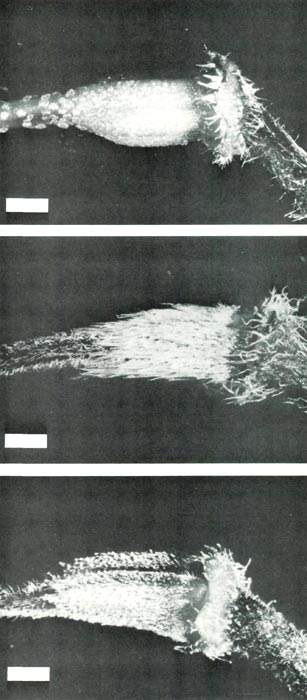
Figure 4: The ovary and base of the
style of (upper, a) R. retusum , showing scales covering the ovary and a few scales at the base of the style; (center, b) R. nudiflorum , showing long pointed hairs densely covering the ovary and short pointed hairs on the style base; (lower, c) R. retusum x R. nudiflorum showing stalked glandular hairs and short pointed hairs on the ovary and short pointed hairs on the base of the style. The bars are equivalent to a length of1 mm. |
The Female Parent
R. retusum
(Bl.) Benn is a tropical
Vireya
species in subsection
Pseudo-vireya
(Sleumer, 1966). It is endemic to the islands of Sumatra and Java in Indonesia and grows terrestrially as a shrub or small tree between 1500 to 3000 m altitude. It is evergreen, as are all species in section
Vireya
, and its ability to withstand frost and air temperature lower than a few degrees below zero is limited. Its seeds are small, and the two "tails" are abnormally short for section
Vireya
(Rouse, 1985a). When selfed or crossed with other Vireyas its seedlings have glabrous cotelydons, and the epicotyl and first leaf are covered with "scales". This juvenile indumentum which lacks hairs is characteristic of all section
Vireya
seedlings which we have examined (Rouse et al., 1984; Williams et al., 1985). In comparison, other lepidote seedlings from sections
Rhododendron
and
Pogonanthum
sometimes have scales on the cotyledons and frequently pointed hairs as well as scales on the first leaf. Leaves from mature plants of
R. retusum
also show only scales on both the upper and lower surfaces of the blade and around the rim. These scales appear translucent on a young leaf, Fig. 1(a), and as the leaf ages they disappear from the upper surface and become dark brown on the lower surface.
The flowers are red and rather small; corolla length 18 mm, tube diameter 4-6 mm and across the lobes 16 mm. The style length including the stigma, and with the stigma just receptive, is 18 mm. The truss usually contains 4 to 8 flowers, Fig. 2(a). The corolla is 5-lobed, the pistil is 5-partite and there are 10 stamens. The base of the corolla and pedicel have a covering of fine straight short pointed hairs with a ring of scales at their junction, Fig. 3(a). The ovary is green and covered with scales which continue a short distance up the style, Fig. 4(a). The flowers are protandrous. At anthesis, the anthers are dehiscing, the style is short and the stigma is small, dry and unreceptive. After anthesis, the style elongates and 8 to 10 days later the stigma, which is now enlarged and well in front of the corolla lobes, becomes receptive. One of the advantages of using this species as the female parent in a pollination where little seed is likely to be set is that if only a few ovules are fertilized these are likely to prevent subsequent abscision of the pistil at the base of the pedicel.
The parent plant used in this cross was introduced into Australia from the Royal Botanic Gardens, Kew, England and grown from a cutting rooted in 1969. The plant is growing outside, root-bound in a 25 cm plastic container in the garden of J.L. Rouse. It flowers profusely for much of the year and is currently nearly 2 m tall.
The Male Parent
R. nudiflorum
(L.) Torrey (syn.
R. peridymenoides
(Machaux) Shinners) is a temperate deciduous azalea in subgenus
Pentanthera
, section
Pentanthera
(Wilson and Rehder, 1921; Bean, 1976; Philipson and Philipson, 1982). It grows in woodlands in the region of the Appalachian Mountains, eastern U.S.A., up to an altitude of about 1200 m, where selected forms are hardy down to -25°C (Galle, 1985). This azalea and
R. viscosum
(also in section
Pentanthera
) were the first species of
Rhododendron
introduced into England from North America, probably about 1730 (Mossman, 1975).
The seeds of
R. nudiflorum
are large with a "wing" (Hedegaard, 1980). Seedlings have glabrous cotyledons, and the first leaf has stalked glandular hairs with occasional pointed hairs on the rim of the blade. This is typical of section
Pentanthera
seedlings which sometimes have pointed hairs on the cotyledons, and pointed hairs or stalked glandular hairs or both on the first leaf (Philipson, 1980; Rouse et al., 1984; Williams et al., 1985). In the mature plant, however, young leaves have only pointed hairs, mainly around the rim, and also scattered sparsely on the upper surface and along the lower surface of the midrib, Fig. 1(b). The flowers are perfumed and the five-lobed corolla has white lobes shading to pink at the base of the tube. The corolla length is 20 mm, the tube diameter is 4 mm and across the lobes is 15 mm. The style length with the stigma just receptive is 40 mm. There are 8 to 13 flowers per truss, and the flowers open in spring before the leaves appear, Fig.2(b). The pistil is 5-partite and there are 5 stamens. The base of the corolla and the pedicel have a covering of pointed hairs, some short and straight, others longer and twisted, Fig. 3(b). The ovary is densely covered with long pointed hairs and the base of the style has short pointed hairs, Fig. 4(b). Flowers are slightly protogynous. The stigma becomes receptive just before anthesis, and as the flower opens, the stigma and stamens protrude in front of the corolla with the stigma in advance as the style straightens and lengthens. A day or so after anthesis the anthers dehisce.
The parent plant used in this cross is about 1 m tall and growing in the garden of J.L. Rouse. It was obtained as seed from the Seed Exchange of the American Rhododendron Society under the number 346 in 1971, with the information that the seed was collected in the wild from a plant on Chapel Hill, North Carolina, by the Azalea Chapter, Atlanta, Georgia.

|
|
Figure 5: The hybrid flower bud, showing pink,
shortly before bud burst. |
The Azaleodendron Hybrid
In September 1981, the pollination
R. retusum
x
R. nudiflorum
was made on two occasions using fresh pollen. The pollinations were made without any special manipulations other than emasculation and bagging of
R. retusum
flowers to prevent contamination with self pollen. Pollinations were then made when the stigmas became receptive. Bags were replaced after pollination and only removed when the stigmas had lost their receptivity. Care was taken to ensure that the
R. nudiflorum
pollen was uncontaminated with foreign pollen. At intervals after pollination pistils were collected, fixed and scored for polled tube growth. Six control pistils were retained on the plant. Seed from these pistils were collected and sown in January 1982. The proportion of filled seed was 38-60% and the overall percentage germination ranged from 4 to 12%. A total of 41 seedlings were obtained, and their hybridity was confirmed a few weeks after the seed germinated when the juvenile indumentum on the first leaf showed the presence of stalked glandular hairs (characteristic of the male parent) and the absence of scales (which would have been present without hairs if the seedlings were accidental selfs or contaminant
Vireya
x
Vireya
). Photographs of typical
Vireya
x azalea seedlings can be seen in Williams et al. (1985). The ability to determine true hybridity of small seedlings obtained from
Vireya
x azalea pollinations was most fortunate, since even seedlings which lacked the vigour to survive for more than a few months could have their true hybridity confirmed. The hybridity of lepidote-elepidote seedlings with the lepidote parent in section
Rhododendron
was similarly confirmed by Martin (1963).
Over the last five years, the hybrid seedlings have been kept in a glasshouse heated to keep the temperature from falling below 10°C, and misted and evaporatively cooled in summer to keep the maximum temperature below 36°C. The seedlings never looked particularly healthy and although their leaves more closely resembled the deciduous leaves of
R. nudiflorum
the seedlings remained evergreen until one by one they died. There are now just three plants remaining. One of these however, has maintained reasonable vigour, producing new growth from the base, and now after 5 years is growing in a 20 cm container and is nearly 0.7 m tall. Its young leaves have stalked glandular hairs on both surfaces and the rim, Fig. 1(c), with a few straight pointed hairs mostly on the upper surface. Early in 1987, a flower bud developed at the top of this bush, and in June some pink appeared in the bud, Fig. 5.
Over a period of 17 days a truss containing 14 flowers opened, Fig. 2(c). The flowers are a blotchy pink speckled with white with a five-lobed corolla and (5) 6 (7) stamens per flower. The corolla length is 27 mm, the diameter at the base of the tube 4 mm, across the corolla lobes 20 mm and the style length is 27 mm. Thus, in its first flowering, the hybrid has produced flowers with a larger corolla and with more flowers in the truss than either of its parents. The base of the corolla and pedicel have a covering of short pointed hairs and a few stalked glandular hairs, Fig. 3(c), the ovary is covered with stalked glandular hairs and short pointed hairs and the base of the style, is covered with short pointed hairs, Fig 4(c). The stigmas became receptive 1 or 2 days after anthesis.
The Fertility of the Hybrid
Pollen of the hybrid was examined microscopically after hydration in 12% (w/v) sucrose solution. The pollen tetrads were shriveled with a mean diameter of 30 m . This can be compared with diameters of 50 to 75 m, the normal range for
Rhododendron
pollen from flowers of this size (Williams and Rouse, 1987.) Examination of stained pollen showed it lacked cytoplasmic content and was therefore sterile.
Backcrosses with fresh pollen of
R. retusum
and stored pollen of
R. nudiflorum
were made to determine the fertility of the hybrid as a female parent and hopefully to obtain seed. Fresh pollen from
R. laetum
was also tried because it was available and
R. laetum
has a style length similar to that of the hybrid. Judging from the effect of relative style length on compatibility in section
Vireya
(William and Rouse, 1988), and assuming that the observations apply to
Rhododendron
in general, incompatibility was not expected due to differences in style length in these pollinations. None of the pollinations resulted in seed and all pistils abscised within two months. Whole pistil squash preparations were made from these excised pistils (Williams et al., 1982) and examined for pollen tube development. Pollen tubes of
R. retusum
and
R. nudiflorum
entered the ovary where they mostly coiled without entering ovules. A few ovule entries were seen with the pollen tubes coiled in the embryo sac but no fertilization was observed. These observations suggest a deficiency in the ability of ovules to interact with normal pollen tubes. Thus it would appear that the hybrid is female sterile. In contrast to the backcrosses, the pollen tubes of
R. laetum
were all arrested in the top half of the style.
Both the parents of the hybrid are diploids (Janaki Ammal, 1950). Since most azaleodendrons with diploid parents are sterile (Kehr, 1977), the complete sterility of the hybrid is not unexpected.
Propagation of the Hybrid
Attempts to root hybrid cuttings have so far been unsuccessful; the cuttings lose their leaves and no roots are obtained. Success, however, might be more likely if cuttings were taken from fairly soft new growth so that the leaves stay in place long enough for roots to form.
Attempts to propagate the hybrid by grafting are, at this stage, inconclusive. While grafts within
Rhododendron
frequently form an initial union which is sometimes successful in the short-term, particularly while there are leaves on the stock, long-term incompatibility may become apparent some years after the stock has lost all its leaves (Rouse, 1985b; Halligan, 1987). Hybrids from section
Vireya
,
R. christianae
x
R. jasminiflorum
, and section
Rhododendron
R. 'Fragrantissimum' were chosen as understock because
R. retusum
appears to be long-term compatible on both, and both root easily producing strong vigorous roots. Grafts formed initial unions, but so far the only short-term success has been with R. 'Fragrantissimum' where one hybrid scion has put out strong new growth. All other grafted hybrid scions have shown leaf abscission within 6 months and no new growth has developed.
Conclusion
It is unfortunate that due to its sterility the hybrid is of no value for further breeding. In addition, it is difficult to propagate and it has unattractive foliage. While we find its flowers attractive they are little if any improvement on those of it parents. We conclude that while we value the novelty of our
Vireya
azaleodendron, it possesses little horticultural potential even if named R. 'Retnud'.
Acknowledgements
We are grateful to Miss Josephine Kenrick for preparing and examining stained pollen, to Dr. Vijay Kaul for examining pollen tubes and to Miss Jill Champ for her skilled assistance in preparing the manuscript. The work was supported by grants to the Plant Cell Biology Research Centre from the Australian Department of Employment, Education and Training, The American Rhododendron Society, the Victorian Branch of the Australian Rhododendron Society and the National Council of the Australian Rhododendron Society.
References
Bean, W.J. 1976. Trees and Shrubs Hardy in the British Islands. Vol. III, 8th Revised Edition, J. Murray, (London).
Galle, F.C. 1985. Azaleas. Timber Press, (Oregon).
Halligan, P. 1987. Delayed graft incompatibility in Maddenii Rhododendrons. J. Am. Rhodo. Soc. 47 (3); 136.
Hedegaard, J. 1980. Morphological studies in the Genus
Rhododendron
dealing with fruits, seeds and seedlings and their associated hairs. G.E.C. Cads Publishing House, (Denmark).
Henslow, C. 1891. Hybrid Rhododendrons. J. Roy, Hort. Soc. 13:240-283.
Heyting, J. 1970. Hybrids between elepidote and lepidote Rhododendrons. Quart. Bul. Am. Rho. Soc. 24 (2); 97-98.
Holttum, R.E. 1941.
Rhododendron
seedlings in Singapore. M.A.H.A. Mag. 77; 93-95.
Janaki Ammal, E.K., Enock, I.C. and Bridgewater, M. 1950. Chromosome numbers in species of
Rhododendron
. Roy. Hort. Soc. Rhodo. Year Book, 78-91.
Kehr, A.E. 1977. Azaleodendron breeding. Quart. Bul. Am. Rhodo. Soc. 31 (4); 226-232.
Martin, A.C. 1963. Rimless scales on lepidote-non lepidote hybrids. Quart. Bul. Am. Rhodo. Soc. 77 (4); 236-240.
Mossman, F.D. 1975. Species Rhododendrons before 1850 - discovery, introduction and classification. Quart. Bul. Am. Rhodo. Soc. 29 (2); 70-77.
Philipson, W.R. 1980. Problems in the classification of the Azalea complex. In Luteyn, J.L. and O'Brien, M.E. (eds). Contributions towards a classification of
Rhododendron
; 53-62. New York Botanical Garden.
Philipson, M.N. and Philipson, W.R. 1982. A preliminary synopsis of the Genus
Rhododendron
III. Notes RBG Edinb. 40 (1);225-227.
Rouse, J.L., Knox, R.B. and Williams, E.W. 1984. Unilateral hybridization in
Rhododendron
. In: "Pollination '84" (eds) E.G. Williams and R.B. Knox, School of Botany, The University of Melbourne; pp. 217-220.
Rouse, J.L. 1985a. The propagation of
Rhododendron
section
Vireya
from seed. Notes RBG Edinb. 43 (1); 99-115.
Rouse, J.L. 1985b. Graft compatibility within section
Vireya
and between this section and section
Rhododendron
. The Rhododendron 24 (3); 43-46.
Rouse, J.L. 1987.
R. retusum
x
R. nudiflorum
in flower. Vireya Vine Newsletter 74.
Sleumer, H.O. 1966. An account of
Rhododendron
in Malesia. Flora Malesiana Ser. 1, 6; 474-668.
Waterson, E.J. 1940. An investigation of the leaf and flower structure of
Rhododendron griersonianum
Balf. f. et Forrest,
R. dalhousiae
Hook. f. and their hybrid R. 'Grierdal'. Trans. Bot. Soc. Edinb.33(1); 1-11.
Williams, E.G., Knox, R.B. and Rouse, J.L. 1982. Pollination subsystems distinguished by pollen tube arrest after incompatible interspecific crosses in
Rhododendron
(Ericaceae). J. Cell. Sci. 53; 255-277.
Williams, E.G., Rouse, J.L. and Knox, R.B. 1985. Barriers to sexual compatibility in
Rhododendron
. Notes RBG Edinb.43 (1);81-98.
Williams, E.G. and Rouse, J.L. 1987. Relationships of pollen size, pistil length and pollen tube growth rates in
Rhododendron
, and their influence on hybridization, (in preparation).
Williams, E.G. and Rouse, J.L. 1988. Disparate style lengths contribute to isolation of species in
Rhododendron
. Aust. J. Bot.36(1)(in press).
Wilson, E.H. and Rehder, A. 1921. A monograph of Azaleas. Arnold Aboretum University Press, (Massachusetts).