The Flowering of Rhododendron 'Arthur's Choice' x R. ovatum: A Vireya x Evergreen Azalea Hybrid
J.L. Rouse
School of Physics
E.G. Williams and R.B. Knox
Plant Cell Biology Research Centre, School of Botany
The University of Melbourne
Parkville, Victoria, Australia
Introduction
The genus
Rhododendron
L. with ca 1000 species is one of the largest angiosperm genera. The species are widespread in the northern hemisphere, from within the arctic circle to just south of the equator, and display an extensive variety of morphological characteristics. Although the genus as currently recognized is paraphyletic and with appropriate minor modifications is probably monophyletic (Kron and Judd, 1990), as might be expected in such a diverse genus, well defined breeding barriers have evolved between major taxonomic subdivisions (Williams et al., 1985) and even within a taxon, barriers have arisen due to disparate flower size (Williams and Rouse, 1988). In particular, there is a strong breeding barrier between the two major subdivisions, the lepidotes (subgen.
Rhododendron
) and the elepidotes (subgen.
Hymenanthes
and the six subgenera in the azalea complex). Among the rare hybrids produced across this barrier, most have been Azaleodendrons with the seed parent in sect.
Rhododendron
and the pollen parent in the azalea complex (Kehr, 1977). Lepidote-elepidote hybrid seedlings have also been obtained with the scaly seed parent from sect.
Vireya
and the pollen parent from the azalea complex. Although the cross between an evergreen Vireya and a deciduous azalea is not one that would be expected to result in viable seed, sect.
Vireya
x sect.
Pentanthera
hybrid seedlings were reported by Williams et al. (1985) and more recently the first flowering of two of them
R. retusum
x
R. periclymenoides
and (R. 'Dr. Herman Sleumer' x
R. herzogii
) x
R. bakeri
was reported by Rouse et al. (1988a, b).

|
Crosses between Vireya and evergreen azalea have been reported by Henslow (1891), Holttum (1941) and Williams et al. (1985). Messrs Veitch & Sons produced the hybrid R. 'Lord Wolseley' x
Azalea indica
1
'Stella'. The seed parent was an F
3
sect.
Vireya
hybrid and the putative pollen parent was in subgen.
Tsutsusi
, section
Tsutsusi
and probably an
R. indicum
or
R. simsii
hybrid. One seedling was flowered, but from the description and sketch of the inflorescence and foliage given by Henslow, it was almost certainly a Vireya x Vireya hybrid. His brief description of a dwarf, slow-growing, sister seedling which died without flowering, suggests it was probably a genuine intersubgeneric hybrid. Holttum crossed
R. longiflorum
(sect.
Vireya
) as seed parent with two varieties of
R. indicum
(sect.
Tsutsusi
) as pollen parents, but the resulting seeds germinated poorly, the seedlings lacked vigour and none survived. We obtained similar results from sect.
Vireya
x sect.
Tsutsusi
crosses. Both (
R. laetum
x
R. aurigeranum
) x
R. indicum
and
R. retusum
x
R. simsii
resulted in small quantities of seed which germinated poorly to produce very weak seedlings whose hybridity was confirmed by the juvenile indumentum on their first leaves prior to their death. We also produced seedlings from crosses between sect.
Vireya
and subgen.
Azaleastrum
:
(a)
R. retusum
x
R. ellipticum
(sect.
Choniastrum
) in October 1981.
(b)
R. lochiae
x
R. championae
(sect.
Choniastrum
) in February 1983, and
(c) R. 'Arthur's Choice' x
R. ovatum
(sect.
Azaleastrum
) in September 1982.
One own rooted seedling of (a) and one grafted onto
R. ellipticum
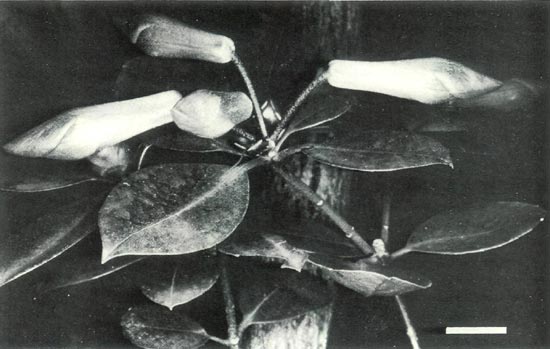
in July 1988 currently survive, but both lack vigour and neither is likely to attain flowering size. There are no surviving seedlings of (b). All the seedlings of (c) died without flowering except for one grafted onto R. 'Fragrantissimum'. Here we report on the flowering of the grafted Vireya x evergreen azalea hybrid (Fig. 1) just prior to its collapse and subsequent death.

|
|
Figure 1: The first flower to open of the
hybrid R. 'Arthur's Choice' x R. ovatum . |
The Lepidote Female Parent, R. 'Arthur's Choice'
R. 'Arthur's Choice' (Fig. 2a) is the F
2
Vireya hybrid (
R. christianae
x
R. lochiae
) selfed, which was raised and named by B. Clancy (Clancy, 1975; Waghorn, 1985). The two parental species are terrestrial Vireyas in sect.
Vireya
, subsect.
Euvireya
, series
Jiavanica
(Sleumer, 1966), and both are diploid (Jones and Brighton, 1972).
R. christianae
Sleum. grows abundantly on steep slopes in the Daga country of New Guinea at an altitude of 500 to 1800 m. It is a rather straggly shrub (to 3 m tall) and has brightly coloured scentless flowers usually deep yellow in the throat with orange shading to red lobes. At the peak of the flowering season, June, hillsides of
R. christianae
produce a striking display of colour clearly visible from an aeroplane (Cruttwell, 1962; Sleumer, 1966). This species was discovered in 1947 and introduced into cultivation by N.E.G. Cruttwell (Cruttwell, 1989). The inflorescence comprises 2-5 flowers, the corolla is 5-lobed and, as with other Vireyas, there is no visible nectar guide. There is, however, a guide for insects in the UV-A, with the corolla tube UV absorbing and the lobes UV reflecting (J.L.R. unpublished). The species is reported to be mainly pollinated by butterflies (Cruttwell, 1989). It is self-compatible and is a parent of many horticulturally successful hybrids, such as R. 'Petra' (
R. christianae
x
R. jasminiflorum
) and R. 'Clare Rouse' (
R. christianae
x
R. laetum
).
R. lochiae
Muell. grows on mountain tops in Queensland, Australia, from Bartle Frere to the north. It was first collected near the summit of Mt. Bellenden-Ker in 1886 on the first ascent by Sayer and Davidson (Sayer, 1888), and was described and named by Ferdinand von Mueller (Mueller, 1887). It is the only Australian rhododendron as yet discovered.
R. lochiae
is a low-growing shrub (to 1 m) with inflorescences of 3-6 bright red, scentless, 5-lobed, tubular flowers. Under UV-A illumination, the face of the corolla is reflecting with a small central absorbing region within the tube. This visible red coloration combined with UV-reflecting lobes around a central UV-absorbing region suggests that in its natural habitat pollination is by birds and/or insects. The rear of the corolla shows an unusual and variable asymmetric patterning (Rouse et al., 1987).
R. lochiae
is self-compatible, and is a parent of many horticulturally important Vireya hybrids such as R. 'Ferdinand von Mueller' (
R. macgregoriae
x
R. lochiae
) and R. 'Liberty Bar' (
R. lochiae
x
R. aurigeranum
). R. 'Little Pioneer' (
R. lochiae
x
R. virgatum
) is a successful sect.
Vireya
x sect.
Rhododendron
hybrid recently reported by Rouse and Blumhardt (1991).
In some respects, R. 'Arthur's Choice' is horticulturally an improvement on both its parental species. It is a delightfully showy floriferous hybrid with bright red unscented flowers (Fig. 2a) larger than those of either parent and leaves that are larger, greener and more leathery. It propagates readily from cuttings and develops a robust root system. It was chosen by the late Arthur Hedlam (well known to readers of this Journal) from among eight seedlings raised from this self-pollination and grown to flowering size.

|
|
Figure 2a: The unscented, 5-lobed flowers of the female parent
R. 'Arthur's Choice', the Vireya hybrid ( R. christianae x R. lochiae ) selfed. There are 10 stamens and no visible nectar guide. The flower on the right shows the top lobe behind its two neighbours and the bottom left hand lobe in front of its two neighbours so this is an example of left-handed chirality. |
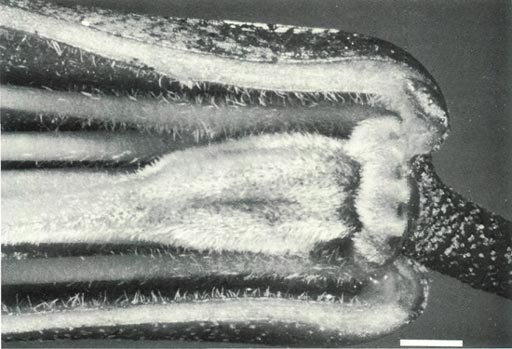
R. 'Arthur's Choice' has Fibonacci phyllotaxis with its leaves grouped in pseudowhorls and the inflorescence is terminal, usually with 3-6 flowers (Fig. 3a). The corolla is bright red, tubular and 5-lobed with no visible nectar guide (Fig. 2a), though under UV-A illumination the face of the corolla has reflecting lobes with a central absorption region (like the parental species), but the absorption region projects onto the base of the top corolla lobe (Fig. 4a). The rear of the corolla shows some increase in absorption on the tube and top lobe, but no sign of the striking well defined UV pattern which is present on the rear of the corolla of R. lochiae . The flowers have 10 stamens, the pistil is 5-partite with a length of 30 mm and a style length of 23 mm. Fig. 5a shows the ovary with a dense covering of fine hairs which hide a mat of scales on its surface, and a tapering ovary-style junction with fine hairs at the base of the style. There are also fine hairs on the inside of the corolla at its base. The outside of the corolla is scaly on the tube and there are scales and a few hairs on the red pedicel.
|
|
Figure 3a: A pseudowhorl of leaves of the female parent
R. 'Arthur's Choice' and six terminal unopen flowers. Scale bar = 1 cm |

|
|
Figure 4a: The face of a flower of R. 'Arthur's Choice'
under UV-A illumination. The corolla lobes reflect the ultraviolet while the central region produces an absorption pattern which extends onto the upper lobe. The pistil and stamens are also absorbing. Scale bar = 1 cm |
|
|
Figure 5a: The ovary, base of the style, nectary, corolla and
pedicel of R. 'Arthur's Choice', showing hairs on the ovary which hide scales on the surface and a tapered ovary-style junction with fine hairs at the base of the style. There are hairs at the base of the filaments and corolla and scales on the pedicel. Sepals are absent from mature flowers. |
Most of the pollen is in non-viable empty shriveled tetrads with a diameter of 33 m, but some 2% are monads (40 m) or diads (50 x 30 m) which appear full and whose size, though rather small, is appropriate for the pistil length (Williams and Rouse, 1990). This fraction of the pollen may be viable. We know of no report of a Vireya hybrid of flowering size with R. 'Arthur's Choice' as a parent, but we obtained vigorous seedlings in 1982 from the pollination R. 'Arthur's Choice' x (
R. laetum
x
R. aurigeranum
) and currently hybrids with
R. gracilentum
♂ and
R. wrightianum
♂ are being raised by B. Clancy (Pers. com.).
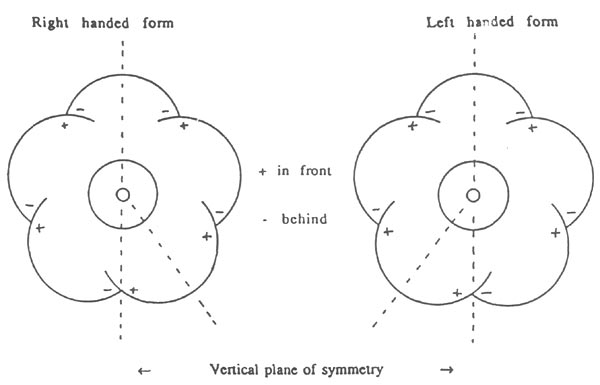
The 5-lobed corollas of R. 'Arthur's Choice' display a chiral symmetry or handedness which depends on the way in which the corolla lobes overlap their neighbours (Rouse, 1990). The top lobe is behind its two neighbours which in turn are behind their neighbours, while the bottom two lobes may overlap either way (Fig. 2a). By definition, the right-hand form (enantiomorph) occurs when the bottom lobe on the right - looking into the face of the flower - is in front of its two neighbours and similarly the left-hand form occurs when the bottom lobe on the left is in front of its neighbours (Fig. 6). The pattern of overlapping of the lobes seldom varies from flower to flower, except for the direction of overlap of the bottom two lobes. In practice, rotation and bending of the pedicel frequently rotate the plane of symmetry away from the vertical. Examination of open flowers with intact corollas in 12 inflorescences gave 22 flowers right-handed, 26 left-handed and 2 indeterminate, suggesting that the two enantiomorphs occur randomly on the bush. However, within an inflorescence, the distribution may well be non-random, as initial observations on the chiral corollas of R. 'Liberty Bar' suggest that inflorescences containing few flowers tend to have flowers of only one handedness.
|
|
Figure 6: The two enantiomorph forms of the chiral 5-lobed corollas
of R. 'Arthur's Choice'. The diagram is applicable to R. ovatum if all the + and - signs are interchanged (i.e. overlaps reversed). |
The Elepidote Male Parent,
R. ovatum
(Lindley) Maxim.
R. ovatum
was introduced into cultivation in England in 1844 by Robert Fortune, who collected it on Chinese Island, China. It is a somewhat variable species which ranges in size from a low shrub to a small tree (to 4 m). It is widely distributed through eastern and central China and also found in Taiwan and Hong Kong.
R. ovatum
is an evergreen azalea, taxonomically placed in subgen.
Azaleastrum
, sect.
Azaleastrum
by Philipson and Philipson (1986) who described it in detail so that only a brief description is given here. Currently, only five somewhat similar species are recognized in this section and their geographical distribution is limited to China and neighbouring regions.
The
R. ovatum
plant used in the cross is growing in the garden of J.L. Rouse and was about 0.5 m tall at the time the cross was made. It was grown from seed collected by P.G. Valder at 200 ft, Ma On Shan, New Territories, Hong Kong in late January, 1975 (collection number 49). The seeds were sown two weeks after collection and the seedlings had 2-4 glandular hairs or pointed hairs on the rim of the cotyledons and 10-14 glandular hairs around the rim of the first leaf.

|
|
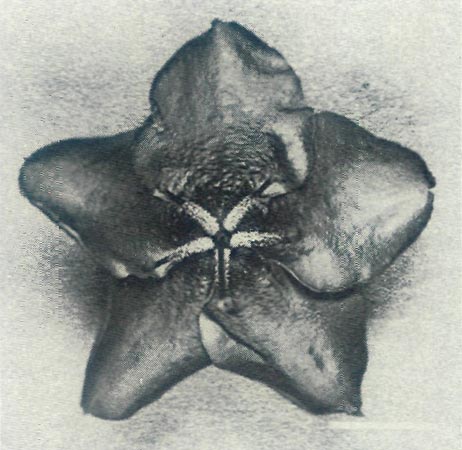
Figure 2b: The unscented 5-lobed flower of the male parent,
R. ovatum with 5 stamens and visible nectar guide on the upper three lobes. The top lobe of the flower is in front of its two neighbours and the lower right hand lobe is behind its two neighbours so this is an example of right-handed chirality. |

|
|
Figure 3b: The lateral unopen flowers of the male parent
R. ovatum
.
Scale bar = 1 cm |
|
|
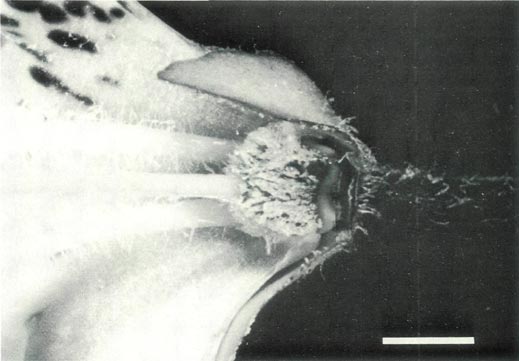
Figure 4b: The face of a flower of
R. ovatum
under
UV-A illumination. The entire face of the corolla and the style are absorbing. The hairs on the stamen filaments are reflecting. Scale bar = 1 cm |
|
|
Figure 5b: The ovary, base of the style, nectary, corolla and
pedicel of R. ovatum , showing glandular and pointed hairs on the ovary, an abrupt ovary-style junction and pointed hairs on the filaments, the large sepals and the pedicel. |
The leaves of R. ovatum are arranged spirally up the stem with Fibonacci phyllotaxis, but the distribution on the stem is far from uniform, bare regions alternating with groups of leaves as in pseudowhorls but with wider spacing. The leaves are glabrous except for a few short fine hairs on the e unscented flower per bud (Fig. 3b). The corolla is 5-lobed, white and slightly saucer shaped with a purple spotted nectar guide primarily on the upper lobe but with a few spots on the two adjacent lobes (Fig. 2b). Under UV-A illumination, the entire face of the corolla is absorbing (Fig. 4b). The flowers have five stamens and the pistil is 5-partite with a length of 24 mm and a style length of 21 mm. Fig. 5b shows the small ovary with a covering of glandular and pointed hairs, an abrupt ovary-style junction, a glabrous style base and pointed hairs on the filament and pedicel. The unornamented seeds are shown in Fig. 7. The pollen is mostly in regular tetrads with a diameter of 66 m, an appropriate size for this pistil length (Williams and Rouse, 1990). The ploidy of only one species in sect. Azaleastrum has been recorded; R. leptothrium is diploid (Janaki Ammal et al., 1950), but it is likely that R. ovatum is also diploid (Janaki Ammal, 1950). We know of no report of hybrids which include R. ovatum as a parent. Further descriptions and colour reproductions of R. ovatum can be found as Azalea ovata in Hooker (1858) and as R. bachii in Hutchinson (1934). Subsequently this latter species has been merged with R. ovatum (Philipson and Philipson, 1986). Descriptions with drawings can be found in Stevenson (1930) and Young and Chong (1980).

|
|
Figure 7: Seeds of
R. ovatum
. They are unornamented like those
of subgen. Tsutsusi . In contrast seeds of subgen. Pentanthera have wings, those of subgen. Azaleastrum sect. Choniastrum have tufts at the ends and those of sect. Vireya have tails. Scale bar = 1mm |
The corolla of R. ovatum displays the same basic chiral symmetry as that of R. 'Arthur's Choice' except that the upper lobe is in front of its two neighbours, which in turn are in front of their next two neighbours (Fig. 2b). As before the bottom two lobes may overlap either way. Examination of the open flowers on the parent plant in the middle of the flowering season gave 22 right-handed and 25 left-handed corollas, again suggesting that on the bush the occurrence of a particular handedness is random.
The Hybrid: R. 'Arthur's Choice' x
R. ovatum
In the period 1981 to 1983, the following pollinations were made:
(a) R. 'Arthur's Choice' x
R. ovatum
,
(b)
R. lochiae
x
R. ovatum
(c)
R. macgregoriae
x
R. ovatum
,
(d)
[(R. macgregoriae
x
R. lochiae
) x
R. macgregoriae
] x
R. ovatum
, and
(e)
R. retusum
x
R. ovatum
Fresh pollen was used for (a) and (d), and for the other pollinations the pollen was taken from a -20°C store. For each cross, the flowers of the female parent were emasculated and bagged to prevent contamination of the stigmas with unwanted pollen, and pollinations were made when the stigmas became receptive. No other special manipulations were employed. Microscopic observation of pistils after pollinations (c), (d) and (e) showed that pollen tubes entered the ovules. Filled seeds were obtained from pollinations (a), (c) and (e), but only those from (a) germinated to produce a few seedlings which showed reasonable initial vigour. Seedling hybridity was confirmed within 5-10 weeks of sowing when the juvenile indumentum on the first true leaf showed the presence of stalked glandular hairs on the rim and an absence of scales (Fig. 8). This ability to determine hybridity of small lepidote-elepidote seedlings is more fortunate, since even seedlings which fail to survive for more than two months can have their hybridity confirmed.
|
|
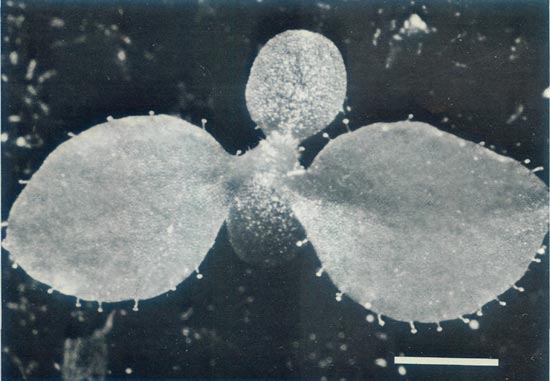
Figure 8: A vigorous seedling of R. 'Arthur's Choice' x
R. ovatum
11 weeks after sowing the seeds. The first leaves have glandular hairs around the rim. This confirms the hybridity of the seedling as glandular hairs are characteristic of the elepidotes and are not found within Vireya. Scale bar = 1mm |
The seedlings were grown on in a greenhouse with heating in the benches to maintain a minimum temperature of 10°C and shading, misting and evaporative cooling to keep maximum summer temperature below 36°C. Ventilation and forced air circulation minimized the build-up of fungal infections. The seedlings, however, developed very slowly, losing their initial vigour and one after the other they died. In June, 1986, three and a half years after the seeds were sown, one of the few remaining seedlings was grafted onto R. 'Fragrantissimum', the stock being rooted at the same time as the union was knitting. This graft combination developed slowly but satisfactorily, and by February 1989, with no leaves remaining on the stock and growing in 150 mm plastic pot, it appeared a compatible combination. However, by June 1990, with the plant 40 cm tall and with four terminals, three of them with flower buds, longer-term graft incompatibility became apparent; the newer leaves were reduced in size, the diameter of the scion just above the union with 40-50% greater than that of the stock just below the union and the stock had become loose in the potting mix indicating lack of rooting vigour. Fig. 3c shows the first flower bud to open about one week prior to anthesis with two un-open flowers, and Fig. 1 shows the first flower to open. The second flower in the same inflorescence opened a week later but thereafter the scion became desiccated and within a few weeks collapsed. The scion and stock were both green and the union was firmly knit but there was minimal root development. While in the short term this graft combination appeared successful, in the long term it was incompatible. This incompatibility may have been exacerbated by the lack of vigour of the hybrid genotype. Our experience shows that frequently, prior to collapse, incompatible grafting induces flowering at an early age and that, no doubt, occurred in this case.
Seedling materials of R. 'Arthur's Choice' x
R. ovatum
were also grafted onto the Vireya hybrid R. 'Liberty Bar' in March 1986 and May 1987 but neither scion flowered. The grafts failed due to incompatibility after 3 years and 1.5 years respectively. Attempts to root cuttings were unsuccessful.
The upper surfaces of the mature leaves of the hybrid were glabrous, with a few glandular hairs on the lower surface, the rim and the petiole. The leaves were arranged in open pseudo-whorls with Fibonacci phyllotaxis, the inflorescences were terminal and the one inflorescence that opened before the plant died was two flowered (Fig. 3c). The funnel-shaped corolla was white with a touch of pink inside, 5-lobed and with no visible vector guide (Fig. 1). The corolla was glabrous except for a few pointed hairs inside near the base, and like
R. ovatum
, under UV-A illumination was absorbing inside and outside (Fig. 4c). The pedicel was finely covered with glandular and pointed hairs. The flower had 8 stamens with pointed hairs at the bottom of the filament and the pistil was 5-partite with a length of 23 mm and a style length of 20 mm. Fig. 5c shows the ovary with a covering of gladular and pointed hairs, an abrupt ovary-style junction and pointed hairs on the style base. The shriveled non-viable pollen was in regular tetrads with a diameter of 35 ± 2 m.
|
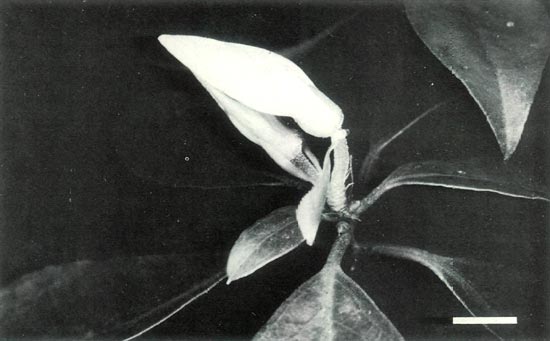
|
Figure 3c: A pair of terminal unopen flowers of the hybrid R.
'Arthur's Choice' x R. ovatum and an open pseudowhorl of leaves. Scale bar = 1 cm |
|
|
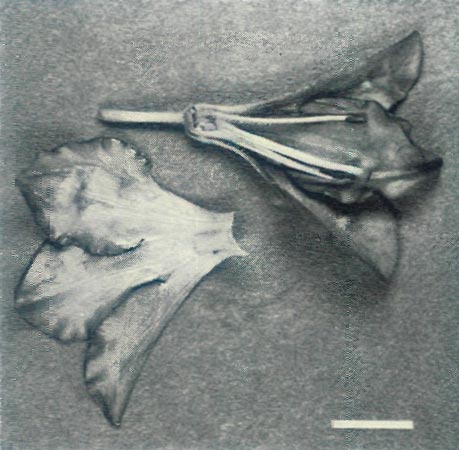
Figure 4c: A dissection of the first open flower of the
hybrid R. 'Arthur's Choice' x R. ovatum illuminated with UV-A. The inside of the corolla is absorbing, while the outside is absorbing on the lobes and reflecting on the tube. The style and filaments are reflecting. Scale bar = 1 cm |
|
|
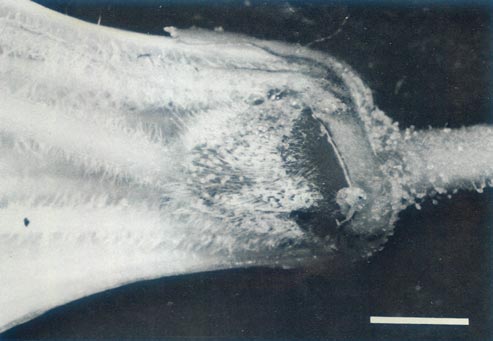
Figure 5c: The ovary, base of style, nectary, corolla and
pedicle of R. 'Arthur's Choice' x R. ovatum , showing the ovary with a covering of glandular and pointed hairs, an abrupt ovary-style junction and pointed hairs on the base of the style and filaments. There are glandular hairs on the sepals and pedicel. |
The second flower to open showed left-handed chiral symmetry of the corolla with the upper lobe in front of its neighbours as in R. ovatum . The first flower to open was either irregular, or the plane of symmetry was displaced from the vertical.
Conclusion
Our experience with Vireya x evergreen azalea hybrids shows that they are difficult to produce, survive poorly and rarely grow to flowering size. With the male parent in sect.
Tsutsusi
seedlings are very weak and the production of a flowering plant unlikely. With the male parent in subgen.
Azaleastrum
, seedlings are marginally more vigorous, and although we have shown that it is possible to produce a plant in flower, much luck was involved, the flowers were sterile and their beauty was decidedly inferior to that of their parents. Our previous reports on Vireya x deciduous azalea hybrids with the male parent in sect.
Pentanthera
have described seedlings with reasonable vigour and more robust plants which grew to flowering size in a reasonable time and are still alive in the garden. The flowers were, however, very distorted, unattractive and sterile.
We conclude that hybrids between tropical Vireya rhododendrons and temperate region azaleas are possible but disappointing. In the exciting but frequently harsh world of reality, any vision of producing hybrids tolerant to extremes of heat and cold which combined the contrasting beauty of the parents into glorious floriferous off springs with the potential for further breeding have evaporated.
Other parental combinations may fare better than ours, but we doubt that significant improvements can be obtained by conventional breeding methods.
Acknowledgements
This work was supported by grants to the Plant Cell Biology Research Centre from the Australian Department of Employment, Education and Training, the American Rhododendron Society, the Victorian Branch of the Australian Rhododendron Society and the National Council of the Australian Rhododendron Society. We thank Miss Jillian Champ for her skilled assistance in preparing the manuscript.
References
Clancy, B. (1975). Malesian Hybrid Breakthrough. The Rhododendron 74 (4): 7-8.
Cruttwell, N.E.C. (1962). Daga Patrol: an account of plant-hunting in the highlands of New Guinea. J. Roy. Hort. Soc. 87: 78-85.
Cruttwell, N.E.C. (1989). Plant hunting in Papua New Guinea with special referent to Rhododendrons in J. Clyde Smith (ed.), Proc. 4th Intl. Rhodo. Conf., University of Wollongong, Australia, October 1988: 96-102.
Henslow, G. (1891). Hybrid rhododendrons. J. Roy. Hort. Soc. 13 (2): 240-283.
Holttum, R.E. (1941).
Rhododendron
seedlings in Singapore. M.A.H.A. Mag. 7 7: 93-95.
Hooker, J.D. (1858). Azalea Ovata. Curtis's Bot. Mag. 84 (whole series), 14 (third series), TAB 5064.
Hutchinson,J. (1934).
Rhododendron bachii
. Curtis's Bot. Mag. 757. TAB 9375.
Janaki Ammal, E.K. (1950). Polyploidy in the genus
Rhododendron
. Roy. Hort. Soc. Rhodo. Yearb. 5: 92-98.
Janaki Ammal, E.K., Enock, I.C. and Bridgewater, M. (1950). Chromosome numbers in species of
Rhododendron
. Roy. Hort. Soc. Rhodo. Yearb. 5: 78-91.
Jones, K. and Brighton, C. (1972). Chromosome numbers of tropical rhododendrons. Kew Bull. 26: 559-561.
Kehr, A.E. (1977). Azaleodendron breeding. Quart. Bull. Am. Rhodo. Soc.
31:
226-232.
Kron, K.A. and Judd, W.S. (1990). Phylogenetic relationships within the Rhodoreae (Ericaceae) with specific comments on the placement of
Ledum.
Syst. Bot.
15
(1): 57-68.
Mueller, F. von (1887). Description of new Australian plants.
Rhododendron lochiae
. Victorian Naturalist 3: 157-158.
Philipson, W.R. and Philipson, M.N. (1986). A revision of
Rhododendron
III. Subgenera Azaleastrum, Mumeazalea, Candidastrum, and Therorhodion. Notes Roy. Bot. Gard., Edinb. 44: 1-23.
Rouse, J.L. (1990). Notes on a selection of Rhododendrons which have flowered during the past year. The Vireya Venture 1: 3-5.
Rouse, J.L. and Blumhardt, O. (1991). Rhododendron 'Little Pioneer': A Vireya-Rhododendron hybrid. J. Amer. Rhodo. Soc. 45 (1): 6-12.
Rouse, J.L, Williams, E.G. and Knox, R.B. (1987). Floral features related to pollination ecology in
Rhododendron
. The Rhododendron 27: 4-6.
Rouse, J.L., Williams, E.G. and Knox, R.B. (1988a). A Vireya azaleodendron in flower. J. Am. Rhodo. Soc. 42 (3): 133-137 and 166-167.
Rouse, J.L. Williams, E.G. and Knox, R.B. (1988b). The flowering of a Vireya x azalea hybrid. The Rhododendron (J. Austral. Rhodo. Soc.) 28 (1): 12-19.
Sayer, W.A. (1888). First ascent of Mount Beilenden-Ker. Victorian Naturalist 4: 32-44.
Sleumer, H.O. (1966). An account of
Rhododendron
in Malesia. Flora Malesiana Ser 1, C.G.J. Van Steenis (ed.) 6 (4): 474-668.
Stevenson, J.B. (ed.) (1930).
The species of Rhododendron
. Royal Horticultural Society, London (2nd ed., 1947).
Waghorn, F.R. (1985). Australian additions to the International Rhododendron Register 1984/85. The Rhododendron 25 (2) 1985: 21.
Williams, E.G. and Rouse, J.L (1988). Disparate style lengths contribute to isolation of species in
Rhododendron
. Austral. J. Bot. 36: 183-191.
Williams, E.G. and Rouse, J.L. (1990). Relationship of pollen size, pistil length and pollen tube growth rates in
Rhododendron
, and their influence on hybridization. Sex. Plant Reprod. 3: 7-1 7.
Williams, E.G., Rouse, J.L. and Knox, R.B (1985). Barriers to sexual compatibility in
Rhododendron
. Notes Roy. Bot. Gard., Edinb. 43: 81-98.
Young, J. and Chong, L.S. (1980). Rhododendrons of China. Binford and Mort, OR. Species descriptions and key from Volume III, Iconographia Cormophytorum Sinicorum, Beijing Botanical Research Institute of Academia Sinica, 1974.