Inter-and Intraspecific Pollinations Involving Rhododendron Species
John L. Rouse, School of Physics
R. Bruce Knox, School of Botany
The University of Melbourne
Parkville, Victoria, Australia
Elizabeth G. Williams
CSIRO, Division of Horticulture
Adelaide, Australia
Introduction
This paper reports the results of inter- and intraspecific pollinations made with
Rhododendron
species and hybrids over an eight-year period from 1980 to 1988. There were a number of reasons for this large crossing program, relating not only to the generation of horticultural hybrids, but also to basic research on breeding barriers within the genus
Rhododendron
and the control of fertilization in plants generally.
With respect to breeding objectives, we wished, if possible, to establish criteria which might assist in forecasting the chances of success in interspecific pollinations. As a result of the associated exploratory crosses, we hoped to generate a number of new hybrids, and to examine the vigour and horticultural potential of these novel plants.
Since taxonomic classifications within
Rhododendron
are by no means settled, we hoped to contribute to this ongoing discussion by providing further data on the strength of breeding barriers between species, sections and subgenera within this very large genus (ca. 1,000 species). Taxonomic separations are normally based primarily on morphological data, but since the ability to exchange genetic material by crossbreeding is important in determining the boundaries of species, exploration of breeding barriers can provide information on the degree of relatedness of various taxa.
Botanically the genus
Rhododendron
is of considerable interest with respect to its reproductive biology (Williams et al., 1990; Palser et al., 1992). Once pollen has germinated on the stigma of the flower, the pollen tubes grow through a mucilaginous secretion in an open pathway from the stigma surface to the embryo sac within the ovule. In contrast to many crop plants in which the time between pollination of the stigma and fertilization within the ovules is very short (a few hours to about two days), in
Rhododendron
this interval is relatively long, being in the order of five days to two weeks depending on the species. This means that the various stages of pollen tube growth through the pistil and their interactions with pistil tissues can be monitored much more precisely in
Rhododendron
than in most other commonly available species. In addition, since the genus
Rhododendron
is large and contains several hierarchical levels of classification (species, subsections, sections, subgenera), there is a broad spectrum of outcomes displayed in interspecific crosses, from fully compatible to unilaterally compatible and completely incompatible. There is also the possibility of crossing with other variously related genera such as
Kalmia
and
Ledum
in the same family (Ericaceae). The genus is therefore well suited to investigation of interspecific incompatibility or "incongruity" (Hogenboom, 1975). An additional positive feature in this respect is the apparent complete lack of a pollen-style self-incompatibility system in
Rhododendron
, since alleles of the
S
gene which normally controls such systems may also affect cross-compatibility between closely related species (Pandey, 1981). In the few instances where self-sterility has been investigated in
Rhododendron
, it has been caused by post-fertilization abortion of fertilized seeds (Williams et al., 1984), not by arrest of pollen tubes in the pistil as normally occurs in
S
-gene controlled incompatibility systems. Our aims in microscopic monitoring of pollen tube growth after pollinations were to gather information on the expression of interspecific incongruity, particularly the time and site of arrest of the normal reproductive process, and to obtain further information on the occurrence of self-sterility within the genus. In this study we placed emphasis on Section Vireya for several reasons: firstly, because it contains nearly one third of the species in the genus, secondly, because its status as a section within Subgenus Rhododendron as opposed to a separate subgenera is still under discussion, and finally, because the Melbourne climate is suitable for cultivation of most Vireya species.

|
|
Figure 1
.
R. hellwigii.
Subgen. Rhododendron, Sect. Vireya, Subsect.
Phaeovireya. This plant was grown from seeds collected by P. Kores on the Finisterre ranges PNG in 1976. It first flowered in 1988 and is self-fertile. More recently, it has been crossed with R. laetum and the selfed and hybrid seedlings are flourishing. |

|
|
Figure 2.
R. aurigeranum
. Subgen. Rhododendron,Sect.Vireya,
Subsect. Euvireya, Series Javanica. One of a number of vireyas introduced into Australia from PNG about 1960. This plant was crossed with R. laetum to produce the outstanding yellow-flowered hybrid R. 'Wattle Bird'. |
Materials and Methods
1. Cultivation of Plants
The plants used in this study were grown by J.L Rouse on his property in Toorak, Melbourne, Australia (latitude 37°51' south, altitude 42 to 44 m and distance from the sea coast of Port Phillip Bay 5 km). Natural climatic conditions measured in the garden over the decade 1980 to 1989 were as follows: Average annual rainfall, 720 + 110 mm (max. 946 mm, min.515 mm). Extreme screen air temperatures for summer, max. 43.5° C, min. 8°C, and for winter, max. 26° C, min. - 0.5° C. In seven out of 10 summers, the daily maximum screen air temperature exceeded 41° C at least once, and in six out of 10 winters the daily minimum screen air temperature was less than 1 ° C at least once. The extreme minimum grass temperature in the open was -6.4° C. Additional climatic data for Melbourne were supplied by the Bureau of Meteorology as follows: For January (hottest month), the average daily maximum and minimum temperatures were 26° C and 14° C, compared with 13° C and 6° C for July (coldest month). Relative humidity was lower in summer than in winter, the average 3 p.m. reading being 43% in January and 62% in July. Since the wet-bulb temperature does not exceed 25° C in Melbourne, plants can be cooled by overhead misting outdoors, and by evaporative coolers in artificial environments.
Seeds were sown on the surface of moist sterile peat moss and germinated in a growth chamber with temperature maintained in the ranges 20° C to 30° C by day and 10° C to 20° C by night, and relative humidity in the range 90% to 100%. A PAR illumination of 70 moles m
-2
s
-1
(4 klux) was supplied for 15-hour days by a mixture of diffuse sunlight, fluorescent tubes and tungsten globes. After three to four weeks when the seeds had germinated, forced-air ventilation was used to lower the relative humidity to 60% for prevention of fungal infections. When seedlings were about 1 cm tall, they were transplanted into a potting mix of sterilized peat moss and pelleted styrene foam with a base fertilizer and watered weekly with dilute liquid fertilizer (Rouse, 1985).
At 5 to 10 cm in height, seedlings were transferred into individual pots in a greenhouse. The greenhouse climate was maintained with electric heating in the benches, evaporative cooling and misting, forced air circulation, control led ventilation and 50% shading in summer. Automatic watering occurred every four to five days. Screen temperatures were maintained between 33° C and 11 ° C in summer and 22° C and 9° C in winter. In the early years of the study, the potting mix was prepared on site from peat moss, pine bark, Isolite (pelleted styrene foam) and fertilizer (Rouse, 1984a), but more recently, newly available, commercial soilless potting mixes, meeting the requirements of Australian Standard 3743, were substituted for the earlier mixes.
Mature plants were grown mostly outdoors in garden beds or containers. Once plants were 30 to 50 cm tall they were transferred to the shade house with automatic misting in hot weather. There they remained, in containers, for two or three growing seasons and, when about 1 m tall, were transferred to the garden. A number of plants were clonally propagated as cuttings, either on their own roots or as scions grafted to more vigorous rootstocks. Rooting of cuttings, with or without grafted scions, took place in the greenhouse in a propagating frame fitted with bottom heat and misting.
Although many
Rhododendron
species from tropical, subtropical and temperate geographic regions(e.g., species in sections Vireya, Rhododendron, Ponticum, Pentanthera, Tsutsusi and Choniastrum) grow, flower and fruit well outdoors in Melbourne, the climate is unsuitable for a number of others, particularly those from high latitudes or altitudes. For example, it has not proved possible to grow
R. camtschaticum
,
R. lapponicum
,
R. ferrugineum
and
R. hirsutum
for sustained periods, and the survival of the tropical but alpine Vireya
R. saxifragoides
is marginal. A few tropical species have been kept in the greenhouse either because they do not survive outdoors (e.g.,
R. intranervatum
)or because of their poor growth (e.g.,
R. gracilentum
).

|
2. Pollinations Hand pollinations were made on plants growing outdoors or in the greenhouse. Wherever possible, crosses were replicated as identical pollinations on at least 10 flowers. In the standard crossing procedure flowers were emasculated by removal of the anthers on the day of opening (anthesis) and were then bagged with clear, finely perforated polystyrene bags to prevent contamination of stigmas with self pollen or insect-borne foreign pollen. For most species, the stigma is not yet receptive at anthesis. Pollinations were made two to four days after anthesis when the presence of a visible exudate on the stigma indicated receptivity. Pollen was applied by touching adehiscing anther to the moist stigma surface. Bags were replaced over the flowers for several days after pollination to ensure that only the desired fertilizations occurred. Because bagging of flowers changes the temperature and humidity in the immediate environment of the pistil and tends to retain water around the flower after rain, bagging may be detrimental to the success of pollinations. For this reason an alternative method for protecting the stigma after pollination was used in the later stages of the crossing program. After pollen had been placed on the stigma, a small square of surgical tape was looped over the top of the stigma and sealed down the sides. This left the flower exposed to natural environmental conditions while shielding the stigma surface from visiting insects and other sources of contaminating pollen. Fresh pollen was generally used for self pollinations, intraspecific pollinations, and interspecific crosses where the species were in flower at the same time. Storage of pollen was required, however, for crosses where the intended parents flowered at different times. Pollen was collected in the anthers, desiccated over silica gel for 48 hours at 4° C, and stored in paper envelopes in sealed glass containers at -20° C (Rouse, 1984b).
3.Assessment of Pollen Tube Growth in the Pistil
For most crosses, several (usually three) pistils were collected at intervals of seven and 14 days after pollination for microscopic examination of pollen tube growth. For some crosses, supplementary samples were taken at four to five days and/or 21 days, and in a few instances samples were taken daily for detailed examination of the rate of tube growth. For all crosses, a number of control pistils were allowed to remain on the plants for seed development. Where any or all of these pistils later abscised, they were also examined microscopically to determine the extent of pollen tube growth before abscission.
Pollen tube growth was assessed by microscopic examination under epifluorescence optics of pistil squashes stained with decolorized aniline blue (Kho and Baer, 1970; Williams et al., 1982). For incompatible crosses, arrest of pollen tubes may occur at a number of sites in the pistil, and may be associated with characteristic abnormalities of the pollen tubes.
4. Seed Production Seeds were collected from all control pistils that produced ripe capsules. After removing the chaff (mostly undeveloped and underdeveloped ovules), seeds were examined for embryo content and then sown. Germination and initial seedling vigour were recorded. Non-vigorous seedlings could sometimes be induced to flower by rooting cuttings or grafting on to vigorous understock (Rouse etal., 1991a).

|
|
Figure 3.
R. veitchianum
. Subgen. Rhododendron, Sect.
Rhododendron, Subsect. Maddenia. This species was grown from seeds collected by P.G. Valder in Thailand in 1975. Our plants seldom set seed. |

|
|
Figure 4.
R. ellipticum
. Subgen., Azaleastrum, Sect. Choniastrum.
Our plants of this species have been raised from seed collected by J. Creech in Taiwan and distributed by the American Rhododendron Society, Seed Exchange in 1969. |
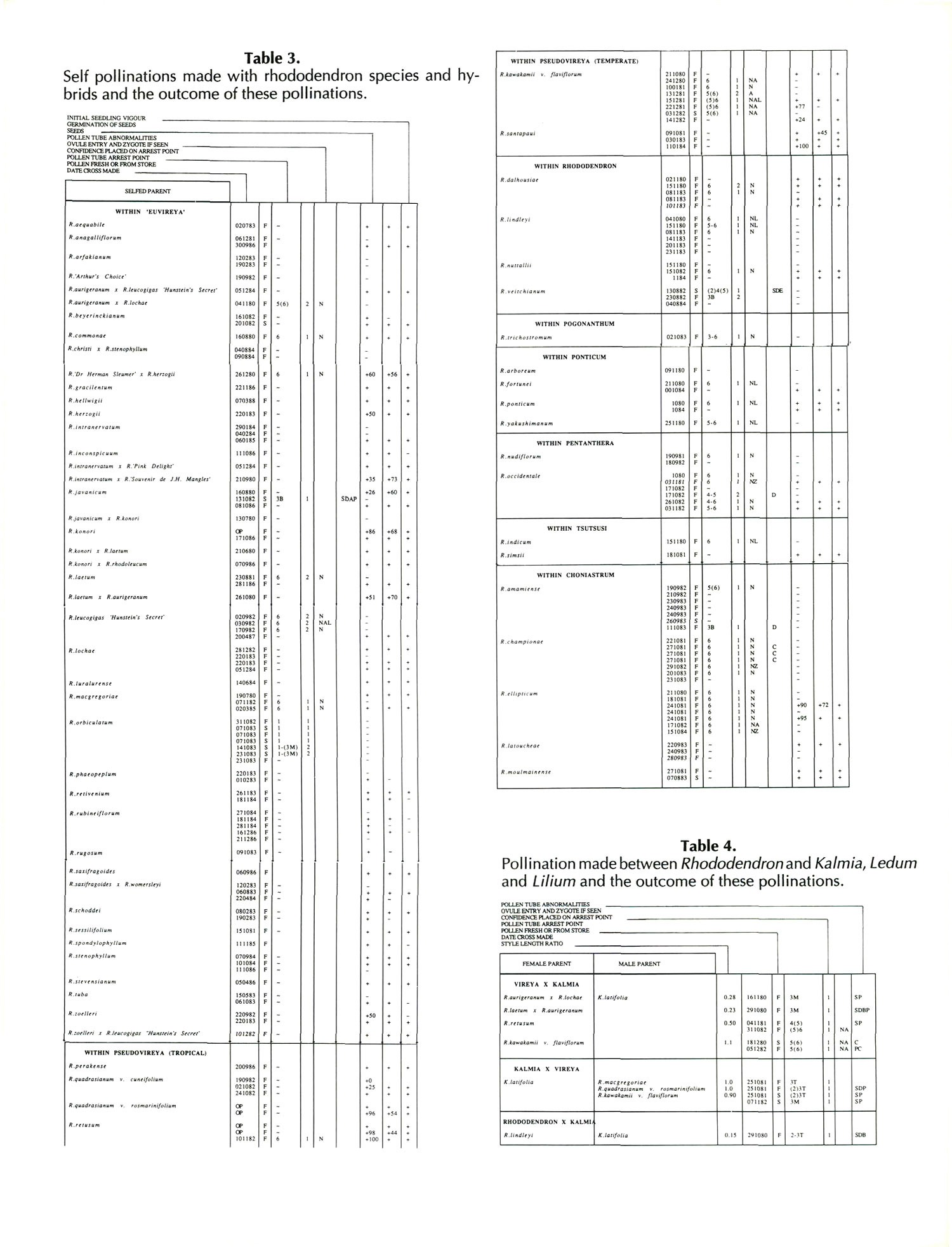
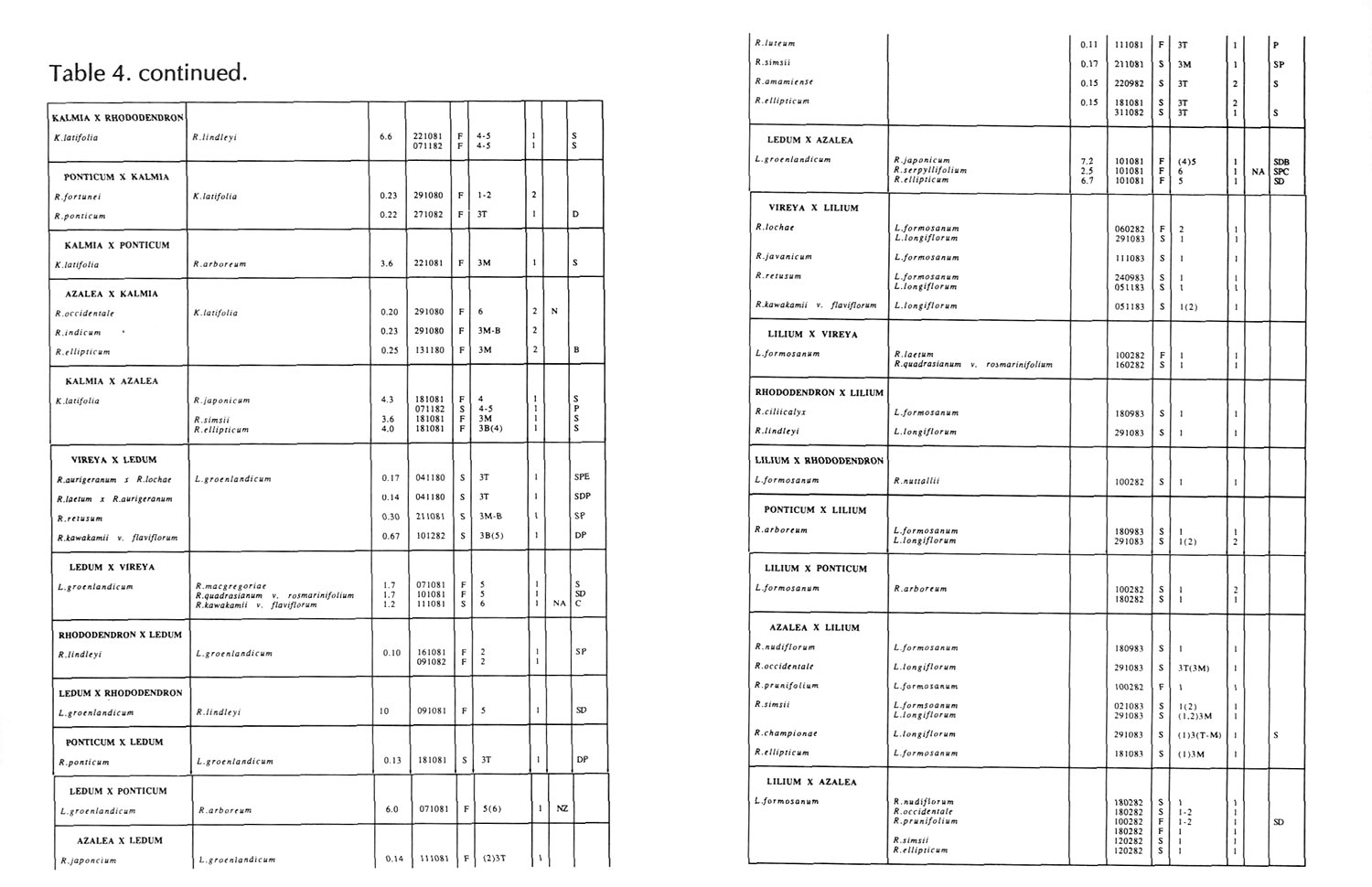
Results and Discussion
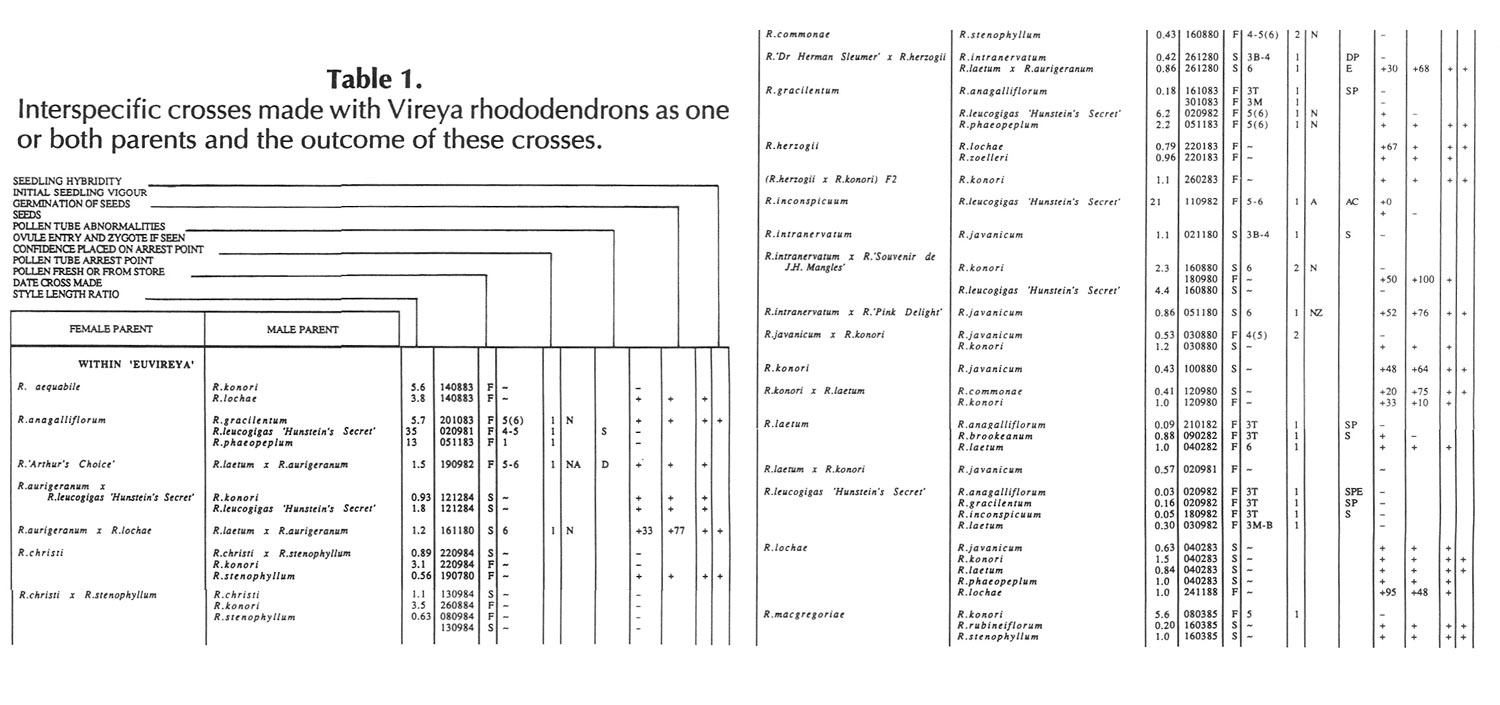
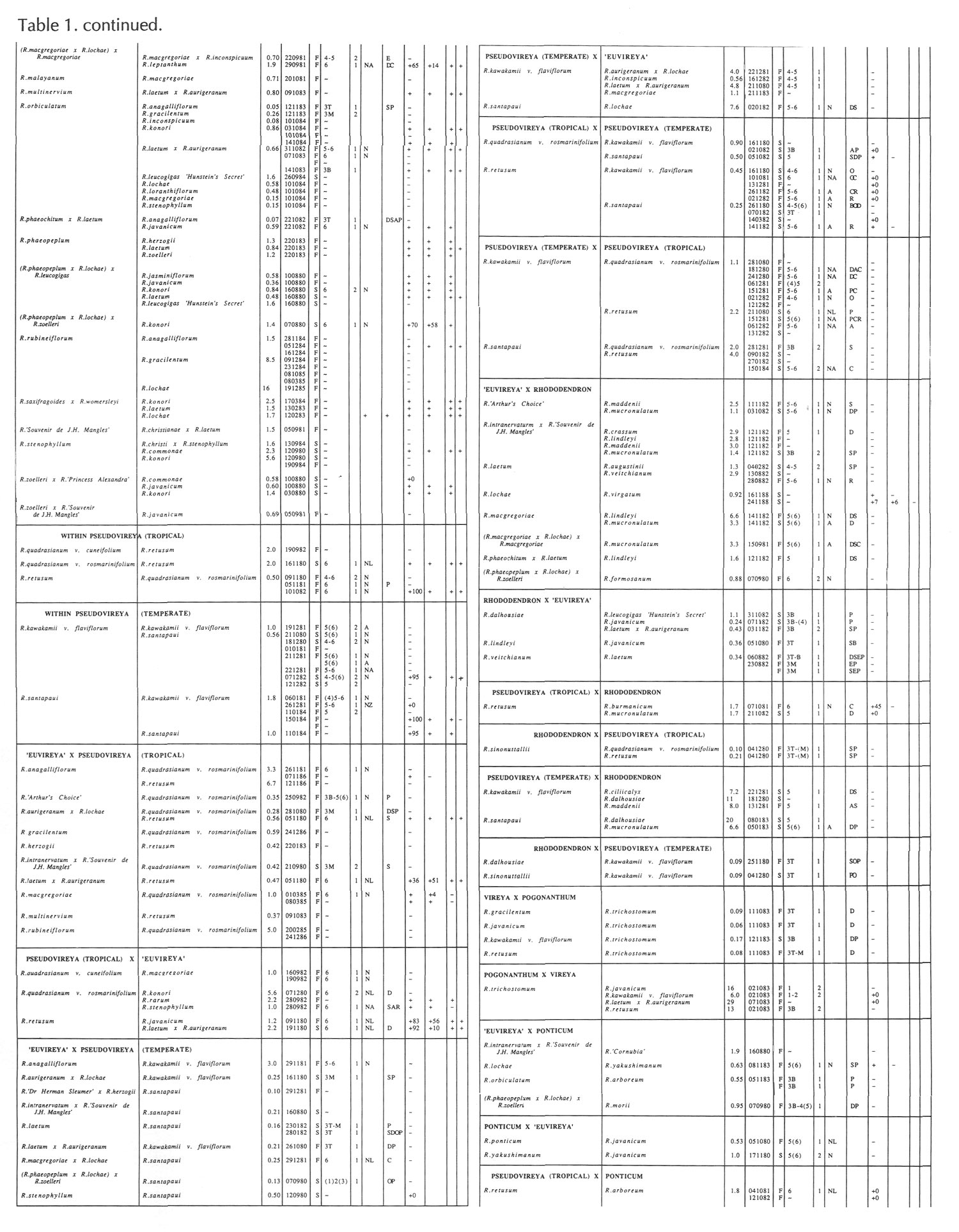
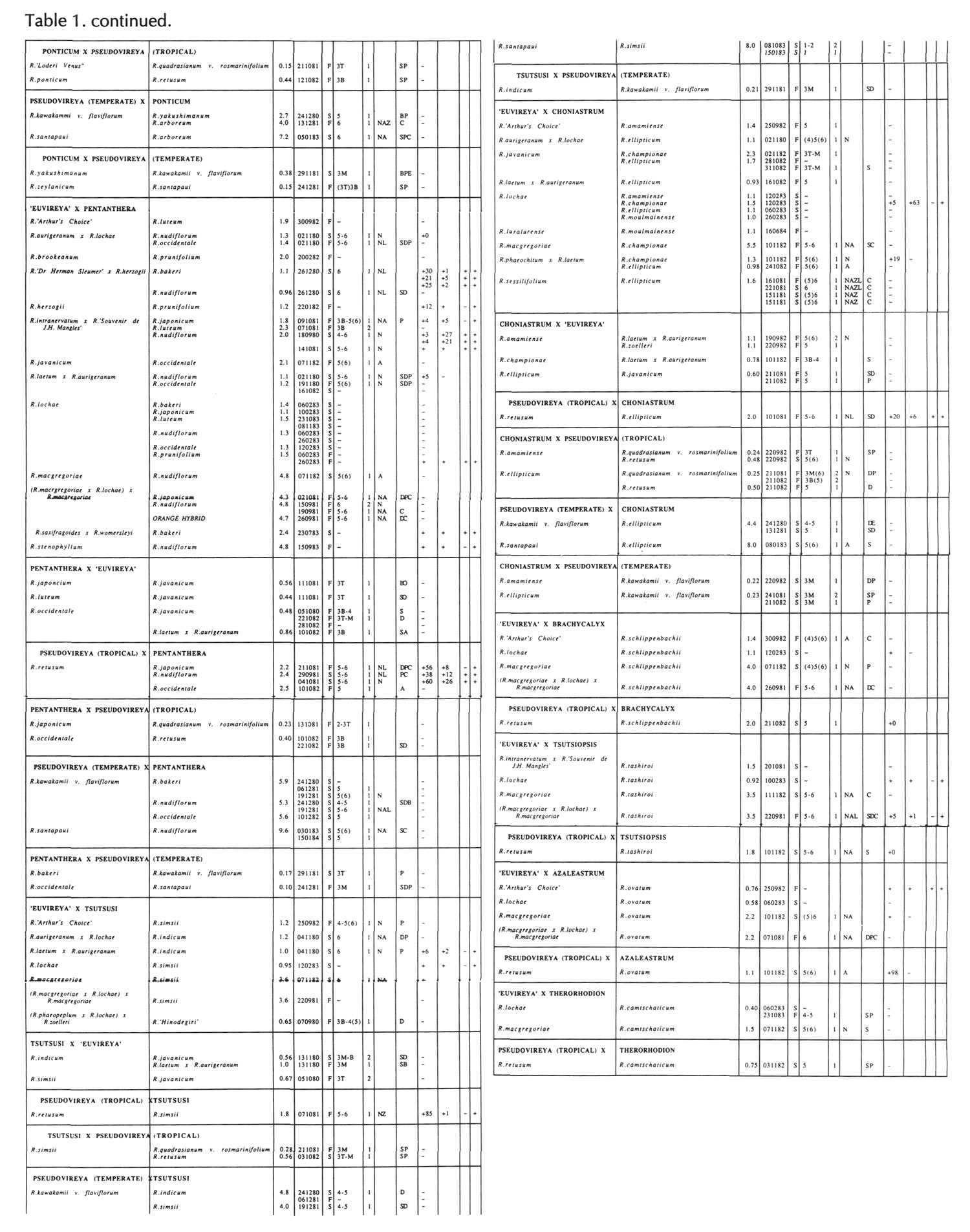
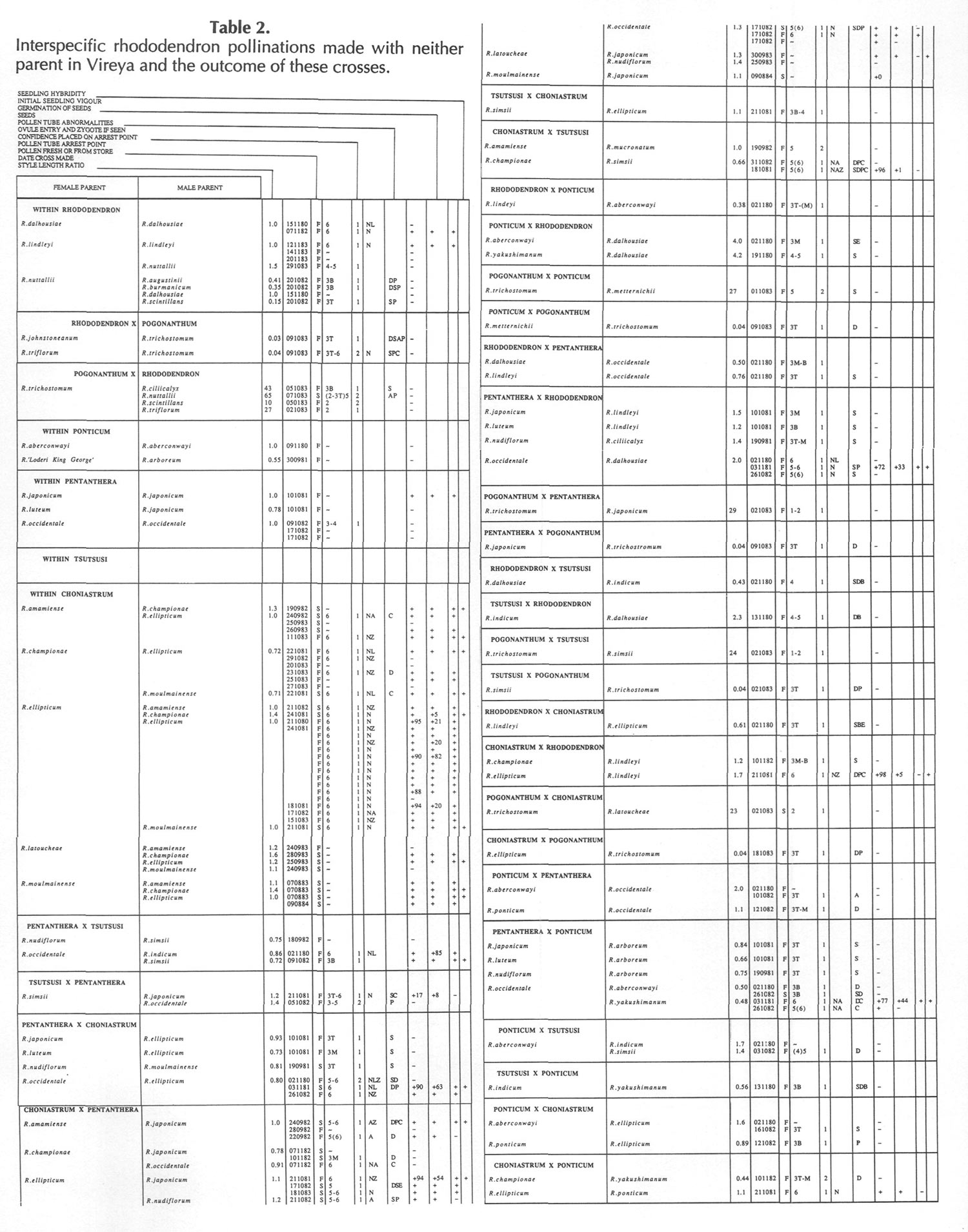
Results of pollinations are given in Tables 1 to 4. The species are grouped in their sections according to Sleumer (1966), but for convenience we have further subdivided Section Vireya into Subsection Pseudovireya and a compound grouping, 'Euvireya', which includes all other subsections. We have also divided Subsection Pseudovireya into species from tropical regions as opposed to those from temperate regions. Table 1 lists interspecific pollinations made with Vireya rhododendrons as one or both parents, Table 2 other interspecific pollinations with
Rhododendron
, Table 3 self-pollinations and Table 4 pollinations made between
Rhododendron
and
Kalmia
,
Ledum
or
Lilium
. Information on the pollination includes the date the cross was made, whether the pollen was fresh or stored, and the ratio of style length of the male parent to style length of the female parent (SLR). Storage of pollen does not appear to influence the outcome of crosses, but sufficiently disparate style lengths have been found to constitute a breeding barrier (Williams and Rouse, 1988).
The tables indicate the extent of pollen tube growth within pistils, and the degree of confidence placed on this arrest point as judged by repeatability in replicate pistils. Low confidence observations have not been included in the tables. In addition, pollen tube growth abnormalities are noted and, if pollen tubes entered the ovules, normal or abnormal entries are recorded and whether zygotes or enlarging ovules were seen. Incompatible pollen-pistil interactions are frequently associated with pollen tube growth abnormalities, usually before but sometimes after entry into the ovules. After compatible pollinations, successful fertilizations can often be distinguished by early formation of a special callose wall around the zygote. Data are also given on the embryo content of seeds obtained, their germinability, and the vigour of any resulting seedlings. The tables also indicate whether hybridity had been confirmed. Such confirmations were generally made by examination of flowers or other mature plant characteristics but, in intersubgeneric crosses between Vireya and the azalea complex, hybridity could be confirmed at five to 10 weeks after sowing by the presence of stalked glandular hairs in the juvenile indumentum on the first foliage leaf. Such hairs contrasted with the scales typical of Vireya seedlings and the simple hairs of azalea seedlings (Rouse et al., 1985).
NOTATION FOR TABLES 1 TO 4
Style Length Ratio
- The ratio of the male parent style length to the female parent style length (SLR).
Date Cross Made
- Day Month Year
Pollen Fresh or from Store
- F fresh, S from store, OP open pollinated
Pollen Tube Arrest Point
|
(Minimum occasional)
(if present) |
Characteristic
arrest point |
(Maximum occasional)
(if present) |
| X - Y = arrest points X and Y and points in between | ||
| ~ arrest point not determined | ||
| arrest points | 0. No pollen or tubes | |
| 1. Pollen present, but no germination | ||
| 2. Tubes abort on stigma surfaces | ||
| 3. Tubes abort in stylar canal | ||
| T Top | ||
| M Middle | ||
| B Bottom | ||
| 4. Tubes abort at the style-ovary junction | ||
| 5. Tubes enter ovary | ||
| 6. Tubes enter ovules | ||
Ovule Entry and Zygote If Seen - N Mostly normal tube entries, A Abnormal tube entries occur frequently, Z Some zygotes seen, L Some ovules enlarged
Pollen Tube Abnormalities
| (i) Tip abnormalities external to the ovule | ||
| S Swollen tips | ||
| D Distorted tips | {abnormally tapered, | |
| {branched, coiled | ||
| {knotted, or otherwise | ||
| {malformed tips | ||
| A Tips end in a heavy callose plug | ||
| B Burst tips | ||
| (ii) Tube abnormalities | ||
| P Tubes lose direction and are | ||
| {undulating, spiraling | ||
| {coiled, knotted, or | ||
| {otherwise not straight | ||
| E Tube diameters are variable | ||
| O Callose abnormalities | {unusually heavy callose | |
| {light, spotty callose in walls | ||
| {unusually short or long plugs | ||
| (iii) Abnormalities within the ovule | ||
| C Coiled tube overgrowths | ||
| R Swollen but intact tube tips | ||
Seeds
+ Seeds were obtained, some of which contained embryos. Number following, if any, is percentage of seeds with embryos.
+0 Seeds were obtained but no embryos were present.
- No seeds were obtained due to pistil abortion.
~ Seeds, if any, were lost.
Germination of Seeds
+ Seeds germinated. Number following, if any, is the percentage germination of seeds containing embryos.
- Seeds failed to germinate.
Initial Seedling Vigour
+ Vigour normal and some seedlings likely to grow into mature plants.
_ Vigour low and most or all of the seedlings unlikely to survive.
Seedling Hybridity
+ Hybridity confirmed from juvenile indumentum, characteristics of mature leaves or by flowering,
left blank: Hybridity unconfirmed.
- Characteristics of the mature leaves and flowers suggest the seedlings are selfed progeny of the female parent.
|
|
|
|
|
|
Incompatibility between species can be expressed during many different phases of the reproductive process or at various stages of the life cycle of progeny resulting from wide hybridizations. Incompatibility barriers may result in empty seeds, failure of seeds to germinate, nonviable seedlings, distortions of the shoot and leaves, plants too weak to flower, or flowers may be sterile. Our crosses within the genus Rhododendron showed a wide variety of outcomes, from complete incompatibility involving arrest of pollen tubes high in the style to full compatibility with production of fertile hybrids. Since we had available only one to a few individuals of any given species and only one or a small number of species to represent many of the taxonomic groupings within Rhododendron , our observations of breeding barriers can be generalized only with caution. For this reason, we will discuss only the major compatibility barriers. Throughout the crossing program, there were occasions when we observed failure of self or cross pollinations that succeeded when duplicated on other dates, in different seasons, or on other plants. This variability probably reflects mostly the effects of inclement weather, the health of the female plant or poor pollen quality. In a few instances, however, it may reflect genetic differences between individual plants of the same species.

|
|
Figure 5.
Ledum groenlandicum
. Our plant was raised from seed
obtained from the American Rhododendron Society, Seed Exchange, in 1973 as Rhododendron lapponicum. |

|
| Figure 6. Lilium formosanum . |

|
| Figure 7. Kalmia latifolia . |
Within Section Vireya, species were generally self-compatible and inter-compatible with important exceptions attributable to two distinct factors: (1) an incompatibility barrier between Subsection Pseudovireya (temperate) and all other species in the genus, and (2) a breeding barrier between species with extremely disparate style lengths, i.e., SLR > 6 or < 0.2 (Williams and Rouse, 1988). Within Psuedovireya (temperate), the cross
R. kawakamii
var.
flaviflorum
x
R. santapaui
resulted in viable seeds and a pollen-sterile flowering plant (female fertility unknown; Rouse etal., 1991b). Our single plant of
R. orbiculatum
proved to be male sterile, but produced hybrids as a female parent when pollinated by
R. konori
. Its female functions are therefore assumed to be normal.
Subgenus Rhododendron, the lepidotes, includes sections Vireya, Rhododendron and Pogonanthum. Although Section Rhododendron contains a more heterogeneous group of species than Section Vireya, we had available only a restricted range, mostly from Subsection Maddenia. Nevertheless, we found an unexpectedly strong barrier between our representatives of Vireya and Rhododendron, our one successful cross being
R. lochiae
x
R. virgatum
(Rouse and Blumhardt, 1991). We were unable to cross our one species in Pogonanthum,
R. trichostomum
, with any other species tested, although this might have been due to its very short style. A strong breeding barrier between Vireya and Rhododendron may be relevant to the discussion of whether Section Vireya should be raised to the level of an independent subgenus (Spethmann, 1987).
The elepidotes include Subgenus Hymenanthes, comprising the single section Ponticum, and the azalea complex which contains a further six sub-genera of which we investigated primarily sections Pentanthera, Tsutsusi and Choniastrum. Generally, species were self-compatible, but in Choniastrum where we have most data,
R. championae
and
R. amamienseare
self-incompatible and
R. ellipticum
produces low seed set when selfed (Williams et al., 1984). While interspecific crosses between the three azalea taxa occasionally produced viable seeds, such seedlings were weak and none survived to flower. Our few results for crosses between the azaleas and Ponticum suggest a breeding barrier which differs in strength depending on the direction of the cross. There is a general trend for the reproductive process to go further when the azalea species is the female parent.
In lepidote x elepidote pollinations our major results are those including a Vireya species. These pollinations also indicate a breeding barrier which differs in its expression depending on the direction of the cross. With Vireya species as pollen parents, no viable seeds were formed and pollen tubes seldom entered the ovules. In reciprocal crosses, however, while pollinations with Ponticum pollen failed to produce seed, Vireya x azalea pollinations occasionally resulted in non-vigorous seedlings some of which eventually produced sterile flowers, e.g.,
R. retusum
x
R. nudiflorum
(Rouse et al., 1988a), (R. 'Dr. Herman Sleumer' x
R. herzogii
) x
R. bakeri
(Rouse et al., 1988b) and R. 'Arthur's Choice' x
R. ovatum
(Rouse et al., 1991a). Unlike Vireya-azalea crosses, the only viable seeds obtained for Section Rhododendron x azalea crosses were with the Rhododendron species as the pollen parent. The resulting seedlings were weak, however, and died before reaching flowering size.
Pollinations between
Rhododendron
species and
Kalmia latifolia
and
Ledum groenlandicum
in related genera within the Ericaceae produced no seed although occasionally pollen tubes entered the ovary or even the ovules. Kron and Judd (1990) have proposed the inclusion of
L. groenlandicum
within the genus
Rhododendron
, and certainly our crosses suggest a breeding barrier no stronger than many of those seen within
Rhododendron
. A number of our pollinations involving
L. groenlandicum
would be expected to fail simply on the basis of overly disparate pistil lengths. Pollinations between
Rhododendron
and the extremely distantly related foreign species
Lilium formosanum
mostly showed failure of pollen to germinate. When germination did occur, pollen tubes usually aborted on the stigma surface and only occasionally entered the style.
Extensive as our crossing program has been, it still represents a restricted and preliminary definition of breeding barriers in this very large and diverse genus. Compared with the number of species in the genus (around 1,000) the range of species in our program is relatively small. In addition, in most instances we had available only one or a small number of plants of each species or hybrid clone. Our results are therefore limited by the fact that the outcome of crosses may vary depending on the individual genotypes of the plants involved, i.e., most but not all individuals of a species may be compatible or incompatible with most but not all individuals of a related species. In spite of these limitations, however, we have demonstrated a number of major and minor barriers which have a bearing on taxonomic relationship within the genus as well as the potential success of horticultural breeding efforts. We hope that our detailed list of pollinations and out comes will provide useful guidance for future hybridizers.
Acknowledgments
This work has received support from the Australian government, the University of Melbourne, the Victorian branch and National Council of the Australian Rhododendron Society, the American Rhododendron Society, and the USA-Australia program of the National Science Foundation. We wish to thank Mrs. Jill Boatman for her expertise in preparation of the tables and manuscript.
References
1. Hogenboom, N.G. (1975) Incompatibility and incongruity: two different mechanisms for the non-functioning of intimate partner relationships.
Proc. Roy Soc. Lond. B
188: 361-375.
2. Kho, Y.O. and Baer, J. (1970) A microscopical research on the incompatibility in the cross
Rhododendron impeditum
x
R. williamsianum
.
Euphytica
79: 303-309.
3. Kron, K.A. and Judd, W.S. (1990) Phylogenetic relationships within the Rhodoreae (Ericaceae) with specific comments on the placement of
Ledum
.
Syst. Bot.
15(1): 57-68.
4. Palser, B.B., Rouse, J.L. and Williams, E.G. (1992) A scanning electron microscopic study of the pollen tube pathway in pistils of
Rhododendron
.
Canad. J. Bot.
70: 1039-1060.
5. Pandey, K.K. (1981) Evolution of unilateral incompatibility in flowering plants: further evidence in favour of twin specificities controlling intra- and interspecific incompatibility.
New Phytol.
89: 705-728.
6. Rouse, J.L. (1984a) Notes on growing media for
Rhododendron
.
J. Am. Rhodo. Soc.
38: 126-132.
7. Rouse, J.L. (1984b) Pollen storage and
Rhododendron
breeding. In: E.G. Williams and R.B. Knox (Eds.), Pollination '84, School of Botany, University of Melbourne, Australia, pp. 185-186.
8. Rouse, J.L.(1985) The propagation of
Rhododendron
section Vireya from seed.
Notes Roy. Bot. Gard., Edinb.
43: 99-115.
9. Rouse, J.L. and Blumhardt, O. (1991)
Rhododendron
'Little Pioneer': A Vireya Rhododendron hybrid.
J. Amer. Rhodo. Soc.
45(1): 6-12.
10. Rouse, J.L., Williams, E.G. and Knox, R.B. (1988a) A Vireya Azaleodendron in flower.
J. Amer. Rhodo. Soc.
42(3): 133-137, 166-167.
11. Rouse, J.L., Williams, E.G. and Knox, R.B. (1988b) The flowering of a Vireya x azalea hybrid.
The Rhododendron
28(1): 12-19.
12. Rouse, J.L., Williams, E.G. and Knox, R.B. (1991a) The flowering of
Rhododendron
'Arthur's Choice' x
R. ovafum
: A Vireya x evergreen azalea.
J. Amer. Rhodo. Soc.
45(4): 218-226.
13. Rouse, J.L., Williams, E.G. and Knox, R.B. (1991 b) The
Rhododendron
hybrid
R. kawakamii
var.
flaviflorum
x
R. santapaui
in flower.
The Rhododendron
31: 16-25.
14. Sleumer, H. (1966) Ericaceae, pp. 469-668 In: Flora Malesiana Ser. 1., Vol. 6(4). C.G.G.J. Van Steenis (ed.) Noordhoff, Groningen.
15. Spethmann, W. (1987) A new infrageneric classification and phylogenetic trends in the genus
Rhododendron
(Ericaceae).
Plant Syst. Evol.
157: 9-31.
16. Williams, E.G. and Rouse, J.L. (1988) Disparate style lengths contribute to isolation of species in
Rhododendron
.
Austral J. Bot.
36: 183-191.
17. Williams, E.G. Knox, R.B. and Rouse, J.L. (1982) Pollination sub-system distinguished by pollen tube arrest after incompatible interspecific crosses in
Rhododendron
(Ericaceae).
J. Cell Sci.
53: 255-277.
18. Williams, E.G., Kaul, V., Rouse, J.L. and Knox, R.B. (1984) Apparent self-incompatibility in
Rhododendron ellipticum
,
R. championae
and
R. amamiense
: A post-zygotic mechanism.
Plant Cell Incompat. Newslett.
16: 10-11.
19. Williams, E.G., Rouse, J.L., Palser, B.F. and Knox, R.B. (1990) Reproductive biology of
Rhododendron
.
Horticultural Reviews
12: 1-67.