Regeneration of Rhododendron PJM Group Plants From Leaf Explants
John E. Preece
1
, Miles R. Imel
2
, A. Shevade
3
Department of Plant and Soil Science
Southern Illinois University, Carbondale, IL
Abstract
A system is described for the regeneration of whole
Rhododendron
PJM group plants from leaves harvested from shoot cultures. Following a two week preconditioning treatment to increase explant survival, leaves were placed on treatment media containing indolebuyricacid (IBA) and isopentenyladenine (2iP) and/or thidiazuron (TDZ). Explant survival increased with increasing concentrations of IBA. Masses of adventitious buds and shoots were larger when 2iP was in the medium, yet TDZ increased the percentage of leaf explants forming shoots and the number of shoots per explant. Microshoots were longer and rooted better when 2iP was the cytokinin. Most plantlets appeared normal; however, some variant plants have been observed when shoots were generated adventitiously. Uses of adventitious regeneration of rhododendrons from leaf explants are discussed.

|
Introduction
Shoots are a combination of stems plus leaves, and adventitious shoots are those that arise at an "unexpected" location such as from leaves or roots. In contrast, axillary shoots form where the bases of leaves are attached to stems (nodes). This is where we normally expect shoots to form as plants branch.
Production of adventitious shoots is not desirable for clonal micro-propagation of plants. Frequently, plants are not "carbon copies" of the original stock plant if their shoots were regenerated from callus or from locations on the plant other than the shoot tip or nodes. There are several reports where growers have complained about the lack of uniformity among micropropagated plants especially rhododendrons (Anonymous 1989, Brand 1992, Knuttel 1987, and Mezitt 1988). A reason for this variability may be that the original shoots were generated adventitiously when the level of cytokinin in the medium was elevated to increase microshoot production and thus profitability.
This variability (called somaclonal variation) is not acceptable to a nursery selling uniform stock that must perform as expected. However, somaclonal variation represents an opportunity to a person interested in selecting and producing new and different plants. If the variation is caused by genetic mutation and is stable and heritable, it has the potential to be used to select new and interesting plants with traits such as resistance to various stresses, including diseases and herbicides; different growth habits; and perhaps different flower colors and shapes.
Adventitious regeneration of shoots from callus, leaves, stems, or flower parts can be used to regenerate plants following the genetic engineering of cells. The potential for genetically transforming rhododendrons using biotechnology is tremendous. Theoretically, genes for desirable traits could be selected from any life form and inserted into rhododendron plants. In the future, one's imagination may be the only limit for choosing useful traits to incorporate into this genus.
Materials and Methods
Explants are the pieces of plant tissue placed in culture. In all experiments our explants were leaf blades (82 mm long and 51 mm wide) cut at their bases away from the node to insure that no preformed buds were present. These leaf explants were harvested from shoot cultures of
Rhododendron
PJM group that were growing on Anderson's (1984) medium gelled with 0.7% Difco Bacto agar and containing 3% sucrose, 50 M 2iP (isopentenyladenine), and 1 M IBA (indolebutyricacid) (Ettinger and Preece 1985).
To increase explant survival from under 50% to nearly 100%, leaves were placed, 10 or more per baby food jar, on Anderson's (1984) medium containing 50 M 2iP and 10M IBA for two weeks (Preece and Imel 1991). Following this preconditioning treatment, explants (Fig. 1) were transferred to culture tubes containing Anderson's (1984) medium and a combination of different concentrations and types of plant growth regulators.
For rooting, microshoots (102 mm long), that formed from the leaf explants, were excised, treated with a 1 M IBA solution for 30 seconds and then placed into a 1 sphagnum moss peat: 2 coarse sand (by volume) medium in a high humidity chamber. The high humidity chamber was placed into a growth chamber. The temperature was 18°C / 25°C (dark/light). Light was provided by cool white fluorescent lights that operated for 16 hours a day.
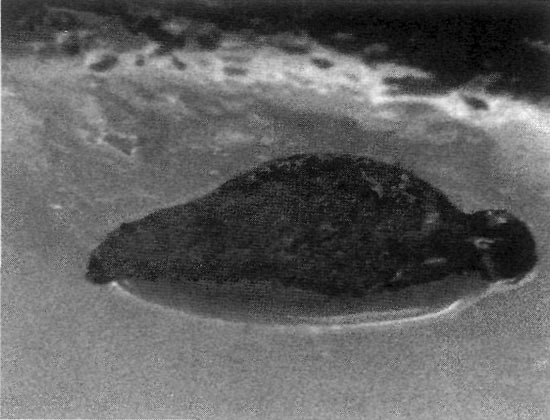
|
|
Figure 1. Leaf explant (1 cm long) after two weeks
on preconditioning medium. |
For acclimatization in the growth chamber, rooted plantlets were transplanted into 6 x 5 x 5 cm (165 cc) rigid polyethylene containers containing the same medium used for rooting. After three to four weeks, they were transplanted into 10 cm standard plastic pots containing pine bark and placed on a greenhouse bench under 30% shade provided by polypropylene shade fabric. The plants were fertilized weekly with 360 ppm N provided by a water soluble 30N-4P-8K (30N-10P 2 O 5 -10K 2 O) fertilizer.
| Table 1. Influence of IBA on the adventitious shoot mass growth (mm 3 ) that formed on leaf explants after 12 weeks on the treatment media | ||||
| Adventitious shoot mass (mm 3 ) a | ||||
| IBA b | 2iP experiment | Thidiazuron experiment | ||
| (M) | volume | n c | volume | n c |
| 1 | 3091.1 | 20 | 2317.4 | 52 |
| 10 | 1447.4 | 42 | 1010.2 | 61 |
| 100 | 256.3 | 53 | 221.6 | 67 |
| Significance | ** | ** | ||
|
**Significant main effect at the 1% level according to the F-test with 2 and 105 df for the main effect of IBA in the 2iP experiment and 2 and 170 df for the main effect of IBA in the TDZ experiment.
a mm 3 (volume)= 1/6 x π x h (h + 3r 2 ). For the purpose of statistical analysis, data were transformed using log10 ((y+1) x 10); non-transformed data are presented. b IBA main effects averaged across 2iP concentrations of 25, 50, and 75 M and TDZ concentrations of 0.1, 1.0, and 10.0 M respectively. c Number of surviving non-contaminated leaf explants. |
||||
Results
BUD AND SHOOT PRODUCTION. We defined shoots as stems with leaves that were at least 0.2 mm long. Buds were shoots that were shorter, had not elongated, and their leaves had not yet expanded (Fig. 2).

|

|
|
| A | B | |
| Figure 2. Adventitious buds (A) and shoots (B) growing from leaf explants. | ||
After 12 weeks on treatment media, buds and shoots were so numerous that they could not be counted without destroying the tissue cultures. The shoot masses were larger when the auxin IBA was combined with the cytokinin 2iP than when the cytokinin was thidiazuron (TDZ) (Table 1). This is a reflection of the inhibitory influence of TDZ on shoot elongation. However, explant survival was considerably higher and nearly twice as many shoots were formed on each leaf in the TDZ experiment than in the 2iP experiment (Table 2), reflecting the beneficial role of TDZ.
Explant survival was higher as the auxin IBA concentration increased, especially when 2iP was the cytokinin (Table 1 [n number increased, indicating increased survival] and Table 2). Unfortunately, accompanying this increased survival with IBA, adventitious shoot number and elongation was reduced. The positive effect of IBA was less when TDZ was the cytokinin.
The tremendous production of short shoots when TDZ was used reflects its great activity as a cytokinin (Table 2). In fact the TDZ was at least 250 times more active than 2iP for stimulating shoot organogenesis. When we tested 2iP at the same low levels as the TDZ, there was no response (unpublished observation). It is common knowledge that cytokinins inhibit shoot elongation at high levels. Shoots elongated better when 2iP was used compared to TDZ (Table 2). Shoots had to be a minimum of 10 mm long to be harvested for rooting because longer shoots were easier to handle.
| Table 2. Influence of IBA, 2iP, and TDZ on adventitious shoot production from Rhododendron PJM group leaves cultured on treatment media for 16 weeks a . | ||||||
| Treatment (M) | Mean number of shoots per leaf explant | |||||
| Shoots | Shoots | |||||
| Number of explants | < 10 mm | > 10mm | ||||
| surviving | with shoots | long | long | |||
| IBA | 2iP | |||||
| 1 | 25 | 7 | 6 | 30.8 | 21.5 | |
| 50 | 6 | 6 | 15.0 | 8.5 | ||
| 75 | 4 | 3 | 23.6 | 25.3 | ||
| 10 | 25 | 10 | 5 | 12.0 | 2.4 | |
| 50 | 18 | 12 | 31.2 | 3.5 | ||
| 75 | 13 | 9 | 25.2 | 6.0 | ||
| Significance b | NS | NS | ||||
| 100 | 25 | 20 | 0 | 0.0 | 0.0 | |
| 50 | 12 | 0 | 0.0 | 0.0 | ||
| 75 | 19 | 1 | 0.1 | 0.0 | ||
| IBA | TDZ | |||||
| 1 | 0.1 | 12 | 10 | 84.8 | 8.3 | |
| 1.0 | 13 | 9 | 87.4 | 1.1 | ||
| 10.0 | 23 | 22 | 56.3 | 0.0 | ||
| 10 | 0.1 | 11 | 6 | 34.8 | 3.5 | |
| 1.0 | 19 | 17 | 49.5 | 0.0 | ||
| 10.0 | 23 | 17 | 50.7 | 0.0 | ||
| 100 | 0.1 | 20 | 2 | 12.5 | 0.0 | |
| 1.0 | 22 | 2 | 17.5 | 0.0 | ||
| 10.0 | 22 | 2 | 5.5 | 0.0 | ||
| Significance b | ** | NS | ||||
| Contrast TDZ c | ||||||
| IBA=1 M | ||||||
| Linear | NS | |||||
| Quadratic | ** | |||||
| IBA = 10 M | ||||||
| Linear | ** | |||||
| Qaudratic | * | |||||
| IBA = 100 M | ||||||
| Linear | NS | |||||
| Quadratic | NS | |||||
|
a
In the original two runs of the experiment, there were 15 leaf explants per treatment per run (30 explants total per treatment), and 22-28 were not contaminated.
b NS Significant interaction at the 1 % (**) level or nonsignificant (NS) according to F-test with 2 and 77 df (2iP experiment) and 4 and 77 df TDZ experiment). For the purpose of analysis, data were transformed using log10 ((y + 1)x 10), nontransformed data are presented. c NS Significant contrast at the 5% level (*), 1% level (*), or nonsignificant (NS) according to F-test with 1 and 77 df. |
||||||
Because we found that TDZ enhanced explant survival and shoot production but inhibited shoot elongation compared to 2iP, we designed an experiment to test combinations of TDZ and 2iP. In this experiment the main effect of TDZ averaged across all three 2iP levels was significant for adventitious bud and shoot number (Table 3). This experiment verified that when TDZ was in the medium a high percent of leaf explants produced adventitious buds plus elongating shoots. Considering that the leaves had been on treatment media for only nine weeks, the nine elongating shoots and nearly 20 buds per explant was high. Because bud and shoot numbers were similar, the presence of TDZ, rather than its level was more important in this experiment.
There was a significant interaction between TDZ and 2iP for the number of adventitious buds that formed per leaf explant (Table 4). The greatest numbers of adventitious buds formed when there was a combination of 1 M TDZ plus 10 or 25 M 2iP, or simply 10 M TDZ alone.
| Table 3. The influence of thidiazuron on callus, adventitious bud, and shoot formation from leaf explants harvested from Rhododendron PJM group shoot cultures after 9 weeks. | ||||||
| Thidiazuron a (M) |
Callus
volume(mm 3 ) |
Number of buds
< 2mm long |
Percent of cultureswith buds |
Number of shoots
(2-5mm) |
Percent of cultures
with shoots |
|
| 0 | 21.0 | 0.4 | 2.4 | 0.2 | 4.8 | |
| 1 | 17.9 | 19.8 | 75.0 | 9.1 | 52.8 | |
| 10 | 12.0 | 15.8 | 86.8 | 9.4 | 65.8 | |
| Significance b | NS | ** | ** | |||
| 5% t-test c | 5.1 | 3.9 | ||||
| 1% t-test | 6.7 | 5.1 | ||||
|
Each mean is based on 36-42 leaf explants.
a Thidiazuron main effect averaged across 0, 10, and 25 M 2iP; all media contained 1 M IBA. b Significant main effect at the 1% level (**) or non-significant (NS) according to F-test with 2 and 107 df. c t-test for paired comparisons. |
||||||
| Table 4. The influence of thidiazuron on callus, adventitious bud, and shoot formation from leaf explants harvested from Rhododendron PJM group shoot cultures after 9 weeks. | ||||||
| Plant growth regulator | ||||||
| Callus volume (mm 3 ) | Number of buds <2 mm long | Percent of cultures with buds |
Number of shoots
(2-5mm) |
Percent of cultures with shoots | ||
| TDZ | 2iP | |||||
| (M) | ||||||
| 0 | 0 | 21.8 | 1.2 | 6.7 | 0.1 | 6.7 |
| 10 | 20.8 | 0.0 | 0.0 | 0.5 | 7.1 | |
| 25 | 20.5 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 1 | 0 | 32.8 | 13.4 | 46.2 | 13.1 | 69.2 |
| 10 | 13.8 | 22.9 | 84.6 | 3.1 | 30.8 | |
| 25 | 3.9 | 24.0 | 100.0 | 10.8 | 70.0 | |
| 10 | 0 | 14.5 | 20.7 | 100.0 | 8.2 | 53.8 |
| 10 | 6.1 | 12.8 | 61.5 | 10.7 | 69.2 | |
| 25 | 15.8 | 13.6 | 100.0 | 9.5 | 75.0 | |
| Significance a | NS | * | NS | |||
| 5% t-test b | 8.3 | |||||
| 1% t-test | 11.0 | |||||
|
Each mean is based on 10-15 leaf explants. All media contained 1 M IBA.
a Significant main effect at the 5% level (*) or non-significant (NS) according to F-test with 4 and 107 df. b t-test for paired comparisons. |
||||||
ROOTING AND ACCLIMATIZATION. Adventitious root formation (Fig. 3) began within three weeks of placing the microshoots into the rooting medium. Thidiazuron in the shoot induction and proliferation medium was inhibitory to subsequent rooting (0 - 73% rooting) compared to 2iP (74 - 100% rooting). The low rooting percentages on shoots that formed on explants exposed to TDZ may be a result of its high cytokinin activity inhibiting root formation.
Observations of rooted plantlets for short periods of time in the greenhouse revealed no outstanding variants (Fig. 4). However, we have observed a few somaclonal variants on adventitiously-derived PJM group plants in the past (Fig. 5).

|
|
Figure 3. Rooted adventitious microshoots. The microshoots were
rooted in a 1: 1 sphagnum moss peat: coarse sand medium. |
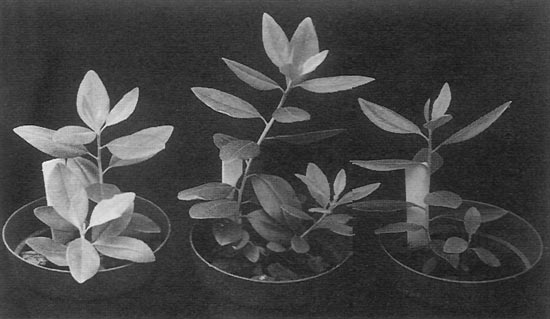
|
|
Figure 4. Plantlets regenerated from adventitious microshoots. While
they are young, most of the plantlets have been uniform. |

|

|
|
| A | B | |
|
Figure 5. (A) Left, "typical" PJM group plant micropropagated via axillary shoot proliferation.
Right and (B) a plant showing somaclonal variation. This plant was dwarf, had a more serrated leaf margin and was heavily branched. |
||
Discussion
Although more adventitious shoots were produced when TDZ was the cytokinin, they did not elongate very well, nor did they root as well as when 2iP was the cytokinin. To improve both rooting and elongation, we transferred masses of adventitious buds and shoots that formed on leaf explants cultured on media with TDZ to medium with 50 M 2iP and 1 M IBA. This was successful. Therefore we recommend a three step process of first preconditioning leaf explants on medium containing IBA and 2iP for two weeks for explant survival, then transferring to medium containing TDZ for 8 to 12 weeks for shoot initiation, followed by transfer to a medium with 2iP for elongation. This has been successful for producing rootable shoots.
It is common in plant tissue culture experiments to detect an interaction among plant growth regulators from different classes, e.g., auxins and cytokinins. There are comparatively few published reports where researchers have combined various concentrations of members of the same class of plant growth regulators. It is interesting that 2iP and TDZ had a statistically significant interaction for adventitious bud formation from leaves of PJM group. Because the two cytokinins caused the same response, one might expect that they acted similarly within the plant. An interaction could indicate that the mechanism by which each compound acted may be different. The chemical structures of the two chemicals is quite dissimilar, and perhaps this, in part, accounts for their interaction. We also have seen an interaction between 2iP and TDZ when flower parts of
Rhododendron
PJM group were cultured in vitro (Shevade and Preece, unpublished).
The ability to efficiently regenerate rhododendrons from leaves has some important applications. The possibility of producing plants that are different because of stable mutations increases if the variants are from adventitious shoots rather than from axillary buds. If horticulturally useful variants can be produced in this manner, they may add additional variety to the selection of rhododendrons. When taking this approach, one must be cautious because there are several causes for somaclonal variation and if it is not stable, plants can grow out of it, reverting to their previous appearance.
Another potential application of this technology is to support genetic engineering studies. If rhododendron leaf cells can be genetically transformed, like other plants, including apple, poplar, and tomato, then the system described here is available to regenerate whole plants from these leaf cells.
Genetic transformation technology has the potential to improve rhododendrons by incorporating important genes, such as those for pest resistance and hormone biosynthesis and control. The benefits of increased pest resistance in rhododendrons should be fairly obvious to anyone growing these plants. However, the potential benefits of being able to insert genes that regulate the hormonal balance may not be as evident. It is the hormone balance that makes plants look like plants. By manipulating the hormonal balance within whole plants, we have the potential to change rhododendron form, size, growth habit, shape, and flowering characteristics. This could be a novel way to improve the genus.
In our laboratory we have been able to use this adventitious regeneration technology to regenerate PJM group from nearly all parts of flower buds and immature flowers (Shevade and Preece, unpublished). We have even been able to determine the influence of cytokinin combinations on flower part (petal) initiation from these flower explants. In vitro flowering has continued in these tissue cultures for more than one year. This is further evidence that scientists may be able to use this technology in the future to both understand and manipulate the flowering process.
Acknowledgements
This research was supported, in part, by a grant from the American Rhododendron Society. We thank Nor-Am Chemical, Wilmington, DE, for providing the thidiazuron, and Sharon Bates, Marta Chytla, Carl Huetteman, and Lynn Long for critical review of the manuscript.
Literature Cited
1. Anderson, W.C. 1984. A revised tissue culture medium for shoot multiplication of rhododendron. J. Amer. Soc. Hort. Sci. 109: 343-347.
2. Anonymous. 1989. Growers spot variations in tissue-cultured stock. Am. Nurseryman. 169(6): 15, 17.
3. Brand, M.H. 1992. Tissue culture variations: Problems. Am. Nurseryman. 175(5): 60-62, 64-65.
4. Ettinger, T.L. and Preece, J.E.1985. Aseptic micropropagation of rhododendron P.J.M. hybrids. Jour. Hort. Sci. 60: 269-274.
5. Knuttel, A.J. and Benoit, L.K. 1987. Growing rhododendrons from tissue culture. Comb. Proc. Intl. Plant Prop. Soc.37: 321-323.
6. Mezitt, R.W. 1988. Abnormal growths on micropropagated rhododendrons. Comb. Proc. Intl. Plant Prop. Soc. 38: 566-570.
7. Preece, J.E. and Imel, M.R. 1991. Plant regeneration from leaf explants of Rhododendron P.J.M. Hybrids. Scientia Horticulturae 48: 159-170.
1
Professor.
2
Former graduate student, Present address: Sherman Nursery Co., Charles City, IA.
3
Former visiting Associate Professor, Present address: School of Studies in Botany, Vikram University, Ujjain MP 456010, India.