Trace Elements in Fertilization of Ericaceous Plants
By R. B. Schaal, Ferro Corporation, Cleveland, Ohio
Talk delivered at the November 11th meeting
of the Middle Atlantic Chapter, American Rhododendron Society
Many diverse aspects of our common interest in azaleas and rhododendrons are represented in the gathering here this evening. Some of you are geneticists, who strive to create more colorful, more shapely varieties or, perhaps hardier and disease resistant strains. Some of you are amateurs, who love to collect and grow those varieties which appeal most to your particular taste and will contribute most to beautifying your homes and grounds. Some of you are commercial growers, whose interest is in making these plants widely available to all who love them. Some of you are plant physiologists or entomologists, interested in protecting and preserving the plants in the course of their growth.
Regardless of these special facets of interest we are all concerned with the problem, "How can our plants be grown to best advantage-how can we be sure they will be as sturdy, as perfect, as prolific and as colorful as possible?"
As industrialists it is our function to help you, as far as we are able, to do this by developing new and better insecticides, growing media and fertilizer through our own research and in cooperation with the State Schools and the Department of Agriculture. An astonishing amount of the manpower and financial resources of some of our land grant colleges is devoted to devising ways and means for fertilizing our soils and making them more productive. In this connection one of the latest subjects to receive attention is the requirement of plants and soils for "trace elements"
In addition to the primary "plant foods," nitrogen, phosphorus and potassium, it is well known that plants need and must have adequate amounts of calcium, magnesium and sulfur to attain proper growth. These three elements are usually referred to as "secondary" plant foods. It has been equally well demonstrated that, to insure proper growth, plants need, in much smaller amounts, iron, manganese, copper, zinc, boron and molybdenum. These six are now commonly referred to as the "minor" or "trace elements." The partial or total absence of one or more of these trace elements may be evidenced in the plants by foliage discoloration, stunting, small size and faded coloring of flowers and by poor varietal shape, color and tastelessness of fruit. I have here a half-dozen slides which will illustrate some of these points. These are all "sand culture" experiments, where the major and secondary elements were added in the form of pure chemicals in adequate amounts, the only treatment difference being either the presence or partial absence of the trace elements. The interesting point here is that in spite of a plentiful supply of the usual fertilizer ingredients in all cases, a deficiency of one or more of the trace elements limits the growth of the plants and produces some or all of the undesirable symptoms which we have already mentioned. A further symptom, in this case lack of boron, is shown by the deficient turnips in the second slide. When these are cut, brown spots are present in the normally white pulp, the turnips are not crisp, but tough, and have a very poor flavor.
As we all know, in their environmental and dietary requirements, plants are rank individualists. The rhododendrons which grow in such profusion on the Welsh coast north of Aberystwyth or on our own coast in the Pacific Northwest would not feel at home in the desert, nor will camellias withstand the bitter winters in the north. Some plants thrive best in the heavy clay soils of the Midwestern prairies, others must have the more open, easily drained and friable soils of our coastal areas. Some plants, such as alfalfa, need plenty of potash and boron, while beans, for example, require much manganese and can tolerate only a moderate amount of boron.
With regard to the azaleas and rhododendrons in which we are particularly interested, most varieties seem to do best in a moderate, moist climate, on comparatively acid, open, easily drained soils of high organic content. Usually a mulch of leaves, peanut hulls or shavings is desirable. Actually, as Mr. Billerbeck
1
has pointed out, a perfectly satisfactory medium for growing azaleas is 100% domestic peat.
While soils of this type are rich in organic nitrogen, they are very apt to be proficient in minerals, especially trace elements. They can easily produce weak and chlorotic plants. While ericaceous plants are not heavy feeders in the sense that tomato plants are heavy feeders, their root systems are adequate to provide, from loose, open, acid soil the moderate nutritional requirements of the plants, provided these requirements are present in available form. The roots are fine, numerous and close to the surface, especially if mulched. They are tender and are easily "burned" by chemicals such as, for example, sodium nitrate or borax. For this reason, if trace elements are needed, care must be exercised in their addition.
In the past trace elements usually have been added to the soil in the form of easily soluble salts. Iron is usually added as ferrous sulfate; manganese, copper and zinc also as sulfates, boron as borax and molybdenum as sodium molybdate. In the presence of moisture enough to dissolve them, these materials are instantly fully available to the plants and can, if present in improper ratios or excessive amounts, cause imbalances or toxicity, which can be as damaging as the deficiencies which we wish to overcome.
If the exact trace element requirement and tolerance of a plant is known and the amount of available trace elements in the soil can be established, it should theoretically be possible to prepare a solution of the soluble salts which would, if added in small amounts at frequent intervals, exactly and ideally fulfill all the plant's needs. Since neither the trace element requirements nor tolerances of most plants, including azaleas and rhododendrons, are known with accuracy and the soil content of available trace elements is exceedingly difficult to establish, even using the best analytical techniques, this method is impractical for field use. It is, in fact, rather impractical for any use except closely controlled greenhouse experiments on a very few well studied crops, in carefully acid washed sand. While the chief hazards presented by the addition of readily soluble trace elements to azaleas and rhododendrons are imbalance and toxicity, their use on other types of soil and on other species of plants presents difficulties equally serious and troublesome. On the light, sandy soils of our coastal plains and lake regions soluble materials are frequently washed from the soil almost completely by a heavy rain and are thus sometimes wasted. Certain of our highly calcareous, alkaline soils precipitate soluble elements from solution in forms unavailable to plants.
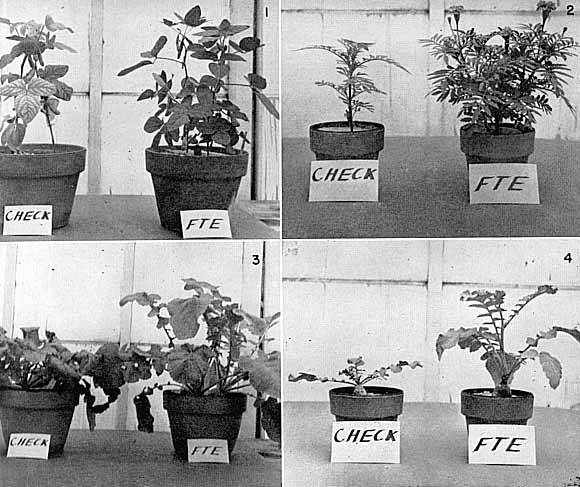
|
|
Fig. 15. Various Plants Grown in Sand Culture with and without the Addition of Trace
Elements. Check Plants--No Trace Elements Added. FTE Plants--Multiple Trace Element Frit added at 100 lbs. per Acre. 1. Upper left--Soy Beans. 2. Upper Right--Marigolds. 3. Loser Left--Turnips. 4. Lower Right--Radishes. |
In short, there would seem to be a place in the fertility program for a trace element material which would overcome these difficulties. This need is now well recognized and is receiving widespread attention.
About ten years ago our Company became interested in this idea. We are primarily manufacturers of porcelain enamel. Certain types of these enamels, or glasses, contain boron, manganese, copper, cobalt, zinc, iron, and molybdenum. We were, therefore, quite familiar with the incorporation of trace elements into glasses. Also we were quite well aware that when these glasses were finely ground they became somewhat soluble in the water in which they were milled. Badger and Bray, working on the problem of solubilizing phosphate rock at the University of Illinois, used a fusion process in their work. This was highly suggestive. It was established that determinations of boron in plant foliage had been ruined by solution of boron from the beakers in which the analysis had been run and, later, one worker showed that enough boron could be leached from finely ground Pyrex glass actually to kill certain plants.
It might be that glasses containing trace elements could be formulated, which, when properly ground, would gradually weather in the soil and yield these trace elements at a slow, fairly uniform rate over a period of time. So, ten years later, after some blood, much sweat and, it must be confessed, not a few tears, we have developed a type of slowly soluble glass, containing iron, manganese, copper, zinc, boron and molybdenum in amounts and proportions which seem adequately to meet the needs of most plants over an entire growing season. We call this product "fritted trace elements," FTE for short. Since it is in the physical form of fine sand it will not translocate or leach from the soil even with violent rains, the contained elements cannot be precipitated in unavailable forms and, since the trace elements in it are slowly released over a comparatively long period, toxicity and imbalances are avoided or minimized, even though enough FTE is applied to last an entire season.
Probably one of the most illustrative examples of the possible advantages obtainable from trace element materials of the type possessing the properties of slow and "controlled" solubility is offered by a tomato experiment conducted last winter and spring in the Horticultural Department at Rutgers University.
2
Tomatoes have, of course, been extensively used as test plants in trace element experimental work and their requirements are fairly well established. They grow rapidly and deficiencies in nutrition are quickly and easily apparent.
The tomatoes in this experiment were grown in acid washed sand and every reasonable precaution was taken to exclude all possible uncontrolled sources of either major, secondary, or trace element nutrition. The major and secondary elements essential to plant growth, nitrogen, phosphorus, potash, calcium, magnesium and sulfur, were added to all plants equally by means of a carefully prepared standard solution in amounts and at intervals judged to be adequate for optimum growth. The plants were divided into six groups.
Group 1, the "check" plants, received only the standard solution.
Group 2, the "control" plants received, in addition, a second standard solution containing the six recognized trace elements; iron, manganese, copper, zinc, boron and molybdenum in amounts and at intervals judged best for optimum growth.
Groups 3, 4, 5, and 6 received no trace element solution but, before the seedlings were placed in the pots, ground FTE frit was mixed with the sand in varying amounts. Through the growth period no trace elements were added to these plants from any other source, the object being, of course, to determine, through measurement of final yield and foliar analysis, whether the single addition of frit at the beginning would supply trace elements to the plants as well as the continued small dosage application of the standard solution.
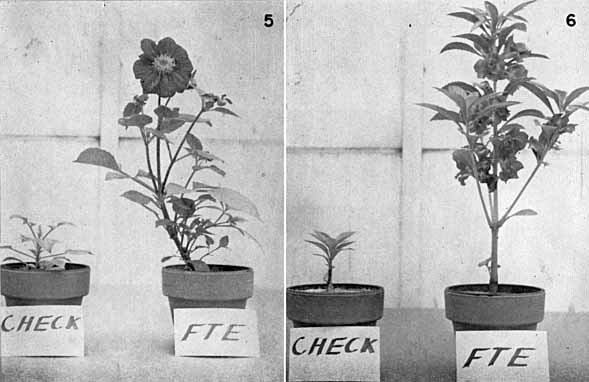
|
|
Fig. 16 Plants Grown in Sand Culture with and without the Addition of Trace Elements.
Check Plants--No Trace Elements Added. FTE Plants--Multiple Trace Element Frit Added at 100 lbs. per Acre. 5. Left--Dwarf Dahlias. 6. Right--Balsam. |
The data, (not yet published) cannot be given here but they will show, in short, that the two frits tested at 100 lbs. per acre, each produced a yield of tomatoes equal to that obtained from the group 2 "control" plants, which had the frequent applications of the standard trace element solution.
This indicates, of course, that the single application of frit was adequate to support the plants throughout the growing season. It becomes especially significant, however, when we consider that, had the total amount of standard trace element solution been added at the start of the experiment, the seedlings might well have been killed or severely damaged from toxicity, or that if, after the trace element solution had all been added, enough distilled water to simulate a heavy rainfall had been poured on and drained off, most of the trace elements probably would have been leached from the pots and the plants therefore, at least partially, "starved."
These two dangers are, of course, greatly modified by the exchange capacity of most soils, especially the high clay soils or organic types, but they exist in varying degrees in most soils and must be provided against if our plants are to do their best.
Since the idea of providing trace elements through the use of glasses of controlled solubility is, perhaps, somewhat novel and the method of making them perhaps unfamiliar, I have here a few slides which will illustrate the manufacturing process used in their production.
The raw materials, which are received in cars, are transported to the fourth floor of the mixing building by a system of conveyors and bucket elevators and are delivered to the distributing conveyor shown in slide (1), which delivers each material to its own particular storage bin rapidly and without danger of error. These storage bins, which will each hold up to two carloads of material, extend down through the third and second floors of the building, their noses protruding through the first floor ceiling as shown in slide (2). Notice the distributor car in the background. The car, which contains, I think, fourteen compartments, is shown receiving materials from the bin noses. Here again we have devices on the delivery gates of the bins and the car compartments which prevent the accidental delivery of a wrong material to the car compartment.
When filled, the electrically driven car moves over a chute delivering into a weighing hopper in the basement. The scale, shown in slide (3) automatically records and prints the weight of the materials delivered from the car to the weighing hopper and here again there is a device which shows which material is being weighed, so that mistakes are eliminated.
When the batch is accurately proportioned, the weighing hopper delivers it by means of a conveyor to the third floor mixer shown in slide (4). The mixer fills and discharges automatically and operates, also automatically, on a definite, predetermined, time cycle. It discharges into a bucket car on the second floor, which is electrically driven to an exact location adjacent to the smelter. Here the batch is dropped into the boot of another bucket elevator which discharges into a bin over one end of the smelter. The bucket car, the elevator and the bottom of this bin, as well as the back of the smelter itself, are shown left to right in slide (5). The bin discharges into a heat and abrasion resistant screw conveyor (just behind the steel column), which gradually and continuously pushes the charge into the back end of the smelter, where it is played on by the flames from a series of burners, melted at about 2400° Fahrenheit into a homogenous glass and discharged through a tap hole, slide (6). The stream of molten glass is met by a two inch stream of water as it falls from the spout. This water shatters the glass into small particles which we call frit. The frit and water fall into a stainless steel tank. The water is re-circulated, while the frit is picked up by a bucket elevator, run through a dryer and delivered to the bin which feeds the grinding mill, slide (7). This mill grinds the frit automatically to a powder of definite particle size distribution and delivers the ground material continuously to a bagging machine, slide (8). The ground frit, in 100 pound multi-wall paper valve bags, is placed on pallets and removed to storage. These pallets, each holding 20 bags, are moved into and out of storage as a unit by a power lift fork truck.
In conclusion it seems to me that, as our knowledge of the requirements of our plants became greater and greater through the researches of our many and highly competent agricultural scientists and technologists, the part played by trace elements in plant nutrition will soon be universally understood and recognized. Certainly the widespread application of this knowledge will result in tastier and more nutritious food for our tables and in more beautiful, more colorful flowers to brighten our lives.
1
W. J. Billerbeck, Appalachian Nurseries, Waynesboro, Pa., In discussion of a previous paper presented at this meeting.
2
Rufus D. Hubbard, Thesis for Master's Degree, Department of Horticulture, Rutgers University, New Brunswick, New Jersey, Jan. 1956.