Breeding For a Purpose
August E. Kehr, Geneticist
Vegetables & Ornamentals Research Branch, Crops Research Division
Agricultural Research Service, U.S. Department of Agriculture
Beltsville, Maryland
Presented at the Annual Meeting of The American Rhododendron Society
Tacoma, Wash., May 14, 1966
Introduction
Only 30 years ago it would have been difficult to find listed in nursery catalogs more than a score of azaleas or rhododendrons developed by American Breeders. Hybrids developed by Barto, Brandt, Gable, Henny, Lancaster, Larson, Lem, Morrison, and Nearing were among the many well-known New World introductions not generally found in Clement Bower's book, first published in 1936. In fact, the situation in Washington, D.C., described in that book, apparently characterized the situation in 1936 for the entire American continent; "Relatively few azaleas or rhododendrons are grown in Washington, D.C., except a few Catawba hybrids,
Rhododendron maximum
, and such of the old Obtusums as 'Amoena' and 'Hinodegiri'" (2). Today through the efforts of many breeders from coast to coast there are hundreds of improved and well adapted forms of American origin of both azaleas and rhododendrons.
The tremendous strides of the last 30 years may well be an indication of the myriad varieties of the future. With the range of variability found in an estimated 800 natural species, few of which have been used in our breeding programs, the potential improvement is almost infinite.
The topic of breeding for a purpose is a broad one, and a speaker could easily take several hours to do it justice. Consequently, I would like to limit this talk to five points which I think are useful ideas or techniques worthy of consideration for incorporation in breeding programs. These points are:
(b) selecting parents;
(c) making and using wide crosses;
(d) colchicine techniques in rhododendron breeding; and
(e) breeding for yellow color.
Collecting and Storing Pollen
Inexpensive and practical techniques are today available to every breeder. They enable him to store rhododendron and azalea pollen almost indefinitely, even to the point of approaching suspended life. Such storage of pollen permits us to make any pollination desired, despite months of time differences in blooming, and despite the fact that continents separate the parents.
Successful pollen storage techniques have been reported for asparagus (14), for various tree fruits (5), and for many other plants including rhododendron (15). Following these methods, I have made successful crosses of deciduous and evergreen azaleas, and scaly and non-scaly rhododendrons, using pollen ranging in age from 1 to 3 years. Briefly, this technique is as follows:
- Collect anthers, preferably just before the flower opens, and put them in labeled gelatin capsules. These are available at most drug stores. Number 1 capsules are about the right size. Avoid large sizes; they are difficult to use in making crosses on small-flowered sorts.
- Pre-dry pollen, within the capsules, for 2-3 days in a home refrigerator. This pre-drying step may be very essential prior to freezing.
- When thoroughly dry, store in sealed jars, over calcium chloride, in the freezer compartment of the refrigerator at about 0° F. (-17° C.). It may be of interest that when stored at -374° F. (-190° C.), the pollen may keep indefinitely (15).

|
|
Fig.31. A simple means of storing pollen.
A layer of calcium chloride or other drying chemical is placed in the bottom of the jar, covered by a layer of cotton, on which are placed the pollen containing capsules. The jars are stored in the freezer compartment of the home freezer. |
To use the stored pollen merely warm the container and make pollinations as needed. The sudden warming does not seem to injure the pollen, neither does repeated thawing and refreezing.
Tapping the capsules almost completely empties the dried anthers of their pollen. The pollen usually adheres to the walls of the vial, making cross-pollination even easier to effect in some cases than with fresh pollen. The dried and stored pollen can be used by inserting the pistils directly into the capsules.
Selecting Parents
Some of you were kind enough to return a survey sheet, listing some of your most successful parents of value in breeding rhododendrons and azaleas. Perhaps it should not be astonishing that there was little agreement among the 33 replies received, including one each from Japan, New Zealand, and West Germany.
One person made the following sage comment which may well describe a goodly proportion of the replies. "Even though I have been hybridizing for some twenty-five years, I feel that I cannot answer your questions with much certainty. There are so many species and hybrids which I have never seen-any one of which may be better than any I list. I have limited my selections to those parents that I have used generally in several crosses and ones which have bloomed several seasons. In five years I might select an entirely different list."
However, there were parental types which were listed over and over. I will briefly list those selected most frequently. These parents are based on summary of 33 questionnaires. The summary includes parents listed three or more times in replies. The author wishes to acknowledge with many thanks helpful information received from the following persons:: Edmond Amateis, Warren E. Berg, Clement Bowers, Dr. Paul J. Bowman, Lester Brandt, Joseph Casadevall, Dr. J. Harold Clarke, Robert Comerford, Leonard Frisbie, Joseph B. Gable, John Henny, Dietrich G. Hobble, Ben F. Lancaster, H. L. Larson, David G. Leach, Halfdan Lem, J. Lofthouse, Dr. Gustav Mehlquist, M. W. Michener, George T. Miller, Ben Nelson, Dr. Carl H. Phetteplace, Dr. A. Frederick Serbin, F. W. Schumacher, A. M. Shammarello, Eugene A. Skonieczny, C. E. Smith, Maurice Sumner, Paul Vossberg, K. Wada, John Waring, Henry R. Yates, and J. S. Yeates.
It appears that hardiness has been one of the uppermost factors considered in these selections. Hardiness is a must for many parts of the country.
| Non Scaly - Species | |||
| # of Replies | # of Replies | ||
| R. catawbiense (selected forms) | 19 | R. elliottii | 6 |
| R. yakushimanum | 15 | R. arboreum | 4 |
| R. fortunei | 13 | R. thomsonii | 3 |
| R. wardii | 8 | R. forrestii | 3 |
| R. williamsianum | 6 | R. haematodes | 3 |
| R. discolor | 4 | ||
| Non Scaly Hybrids - Cultivar | |||
| 'Boule de Neige' | 6 | 'Mars' | 3 |
| 'Loderi Hybrids' | 5 | 'Fabia' | 3 |
| 'Lady Bessborough' | 4 |
'Margaret Dunn' |
3 |
|
'Naomi' |
3 | ||
| Scaly - Species | |||
| R. carolinianum | 14 | R. russatum | 5 |
| R. racemosum | 11 | R. moupinense | 5 |
| R. ciliatum | 8 | R. bullatum | 4 |
| R. cinnabarinum | 7 | R. carolinianum album | 4 |
| R. dauricum | 7 | R. concatenans | 3 |
| R. mucronulatum | 7 | R. minus | 3 |
| R. augustinii | 7 | R. impeditum | 3 |
| R. keiskei | 5 | R. mucronulatum (Cornell Pink) | 3 |
| Scaly Hybrids - Cultivars | |||
| 'Blue Diamond' | 3 | 'Lady Chamberlain' | |
| 'Russautinii' | 3 | ||
| Deciduous Azaleas - Species | |||
| R. calendulaceum | 9 | R. arborescens | 5 |
| R. occidentale | 9 | R. roseum | 4 |
| R. bakeri | 6 | R. luteum | 3 |
| Deciduous Azaleas - Hybrid Cultivars | |||
| 'Klondyke' | 3 | 'Cecile' | 3 |
| Disease Resistance - Species | |||
| R. catawbiense | 4 | ||
In my garden, powdery mildew is a severe problem, especially on seedlings and deciduous azaleas. 'Persil' is the only deciduous azalea that I have found to be highly resistant. R. occidentale is extremely susceptible; the other American species are only moderately resistant.
| Insect Resistance - Species | |||
| # of Replies | # of Replies | ||
| R. fortunei | 6 | R. yakushimanum | 3 |
Those with indumentum were reported to resist insects. 'Boule de Neige' was reported to be very susceptible to insects.
| Cold Resistance - Species | |||
| # of Replies | # of Replies | ||
| R. catawbiense | 17 | R. smirnowii | 5 |
| (including selected forms) | R. carolinianum | 4 | |
| R. yakushimanum | 7 | R. maximum | 3 |
| Heat Tolerance - Species | # of Replies |
| R. catawbiense (all forms) | 3 |
On the basis of some of my experience I would add R. yakushimanum as a source of heat tolerance.
Wide Crosses
Some plant breeders seldom try radically wide crosses. Yet in my judgment such wide crosses offer opportunities unlimited in creating new and radically different forms of plants. Wide crosses offer a stimulating challenge, and we should all occasionally try crosses ranging beyond the species or even genus.
I have a deep admiration for that pioneering spirit who, accidentally or purposely, crossed the elepidote rhododendron
R. griersonianum
with the lepidote
R. dalhousiae
and came up with the hybrid, 'Grierdal' The author would deeply appreciate hearing from persons with first-hand experience with this plant.
In like manner, the interesting lepidote-non lepidote hybrids of Martin (9) represent the type of venturesome crosses that we should all try whenever there is opportunity. Wide crosses probably have little value in themselves. It is the recombinant types which may appear in later generations that have huge potential.
The first recorded rhododendron hybrid was an azaleodendron. It came from an accidental cross, made in 1800, between the broad-leaved
R. ponticum
and the deciduous azalea,
R. nudiflor
um. (2). Bowers makes this meaningful comment: "Probably no hybridist of the time would have dreamed of attempting so wide a cross if this first azaleodendron mating had not occurred accidentally." There may be persons who believe little value is gained from azaleodendron breeding. However, experience with other plants indicates that valuable new types of rhododendrons could result from this kind of cross.
Crosses between the Asiatic and American species and the Javanicums should be encouraged, even though to date few successful crosses have been reported. These species represent germ plasm that has been too little used in rhododendron breeding to date.
|
|
||||
|
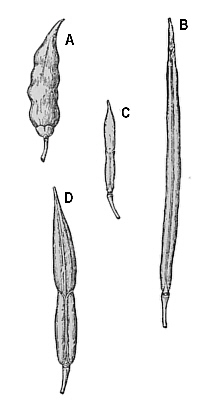
Fig. 33 (left). Example of the effects of a wide cross between radishes and cabbage. A -
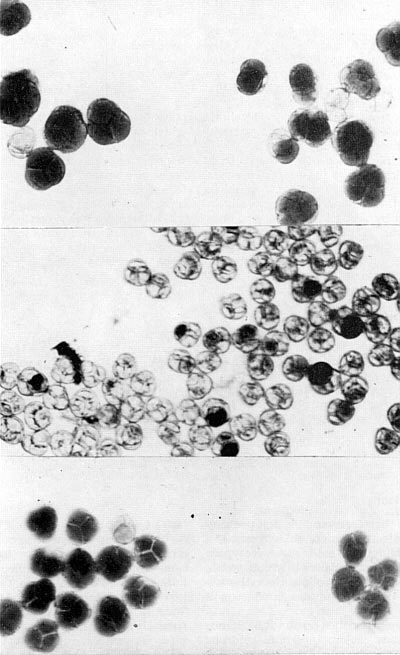
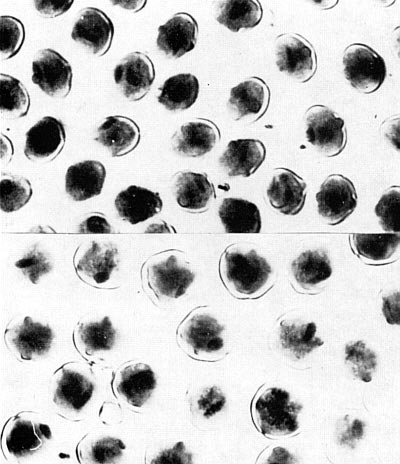
full seed capsule of radish. B - full seed capsule of cabbage. C - empty seed capsule (no seed) in sterile and un-doubled hybrid. D - full seed capsule in doubled hybrid. This is one of the classic examples or restoring fertility by doubling. Fig 34 (right). Pollen of a doubled and un-doubled plant. Top un-doubled, bottom doubled. Doubled pollen has twice the volume of un-doubled pollen. The examination and comparison of pollen size is one of the easiest means of determining whether the plant has been doubled. Fig. 34 Photo by Dermen |
|||||
|
|||||
|
|||||
Colchicine Techniques in Rhododendron Breeding
Many of you may he familiar with doubled forms of ornamentals. The flowers are larger, thicker textured, and longer lasting than most un-doubled forms. 'Gargantua', a form of
R. diaprepes
, has 39 chromosomes rather than 26 (3). This form differs from the normal species in having larger flowers and leaves. Likewise the tetraploid or doubled clones of azaleas 'Tahei', 'Banka', and 'Wako', reportedly have 52 chromosomes rather than the usual 26 (6). They likewise have large flowers of unusual heavy texture. For this reason these new azaleas represent valuable new germ plasm that should be used by every azalea breeder.
Chromosome numbers in the genus Rhododendron range from 26 for most species to 156 for one form of R.
manipurense
. (3). Of the American species only
R. calendulaceum
and
R. canadense
possess more than 26 chromosomes. Because
R. calendulaceum
was used so widely in the Exbury and Knaphill hybrids, it is very probable that some of these hybrids, especially those with the larger-sized flowers, possess more than the normal 26 chromosomes.
The technique of doubling chromosomes by using colchicine, has been known for 30 years. Yet despite the potential which it offers to produce beautiful and desirable types, it is unfortunate that rhododendron fanciers have not used it more widely.
The recommended treatment for treating woody plants, as taken from a USDA publication (4), is as follows:

|
|
Fig. 37. Treating a woody plant with colchicine solution.
The stem is cut back to force rapid growth. The treated buds are later forced to grow by removing the growing tip. Photo by Dermen |
"Cut off the very tip of a vigorously growing shoot and wet with aqueous solution of colchicine (0.5 to 1.0 percent colchicine in a 10 percent water solution of glycerin) the upper three lateral buds, three to eight times at 1 or 2 day intervals. Other buds either should be dug out or shoots growing from untreated buds should be destroyed. Colchicine effect should be looked for in those buds or shoots that are partly retarded in growth as a result of treatment. The effectiveness of the treatment may be judged from the distorted growth of the first few leaves emerging after the treatment. If no such distortion is observed in the lower leaves of the growing shoot, it is most obvious that colchicine solution has not penetrated into the treated buds at all. When fully doubled, growth is no longer distorted. The distortion is usually the result of a mixture of doubled and un-doubled tissues.
"It is advisable to limit treatment to buds of one young shoot on a cutting or grafted shoot, the rest should be cut off. In woody material total polyploidy affecting the whole new growth very seldom occurs. The effect may be confined to a narrow sector on a branch, or to a certain tissue, or to a limited portion of tissues."
Warning:
In handling colchicine, extreme care should be taken to keep it out of eyes to avoid possible dangerous consequence. Wash hands after contact with the chemical to prevent possible skin irritation.
Do not take internally even in very minute amounts. Colchicine is an alkaloid similar to nicotine and is very poisonous even in small doses.
Because of its remarkable potential, the colchicine technique is a tool that should be used by every rhododendron breeder. Its use is especially encouraged for azaleodendrons and first generation hybrids from wide crosses to provide fertile types that can be used in further breeding. The technique is simple and can be used by anyone. It only requires patience, persistence, and careful observation. Some of us in the East are trying our luck with this method, and there are some promising indications of successful doubling in two species and one hybrid, the latter by George Miller of Hanover, Pennsylvania.
Breeding for Yellow Color
Quite satisfactory yellow forms already exist in deciduous azaleas, and to lesser degree, in the scaly rhododendrons. However, a yellow of any hue is lacking in evergreen azaleas, and deep yellows are still the goal of hundreds of breeders of the broadleaved, non-scaly rhododendrons. The following discussion will therefore deal only with the latter two groups.
Broad-Leaved types:
There are many rhododendron breeders who have a goal of developing new types with dandelion yellow color. This is no idle dream, but its fulfillment will not be fast in coming unless we broaden our present methods and materials. There are many sources of yellow genes which to my knowledge are not being used today.
For example, yellow or cream colored forms occur in 75 non-scaly species in 13 series. I have estimated that today at best we are using only 15 species from five series. Good yellows will undoubtedly result from pyramiding genes from many sources.
The inheritance of yellow color in rhododendrons is unknown. However, evidence from many crosses would indicate that yellow is not generally inherited by a dominant gene or genes. However, in crosses between the pink
R. williamsianum
x
R. campylocarpum
, the yellow of the campylocarpum parent is dominant over the pink. How else would we have the yellow-flowered 'Moonstone'?
Furthermore, within a yellow flowered species such as
R. campylocarpum
, there are various shades of yellow ranging from cream color to an intense light yellow (8). For such variability one must postulate that more than one gene controls yellow color. These may be intensified genes with an additive effect, or they may be suppressor genes that diminish color intensity.
Dr. Yeates of New Zealand has suggested that one may obtain fullest expression of yellow genes through the use of yellow hybrids rather than the original species. From a genetic standpoint this is a means of avoiding genes which dilute the yellow color. In roses deepest yellow color seems to be obtained when suppressor and modifier genes are in the recessive condition (10). Rhododendron breeders may get some consolation from the fact that the intense yellow color of roses all stems from a cross made in 1900 by J. Pernet between the rose variety 'Antoine Ducher' and a seedling of 'Persian Yellow' (11). The 'Persian Yellow' had been introduced into France from Iran in 1788; thus it took over a hundred years for someone to find the right parent. (13)
Another fact from the inheritance of yellow color in roses may be significant. The intensity of yellow color was greater in tetraploid (doubled) forms than in diploid (un-doubled) forms, (10), as follows:
| Diploid and Tetraploid Color Intensity | |||||
| Classes for Yellow | |||||
| Pale | Light | Medium | Dark | Intense | |
| Diploid | x | x | x | ||
| Tetraploid | x | x | x | x | x |
It is therefore possible for progeny to be selected from doubled forms of a good yellow such as
R. campylocarpum
or 'Goldsworth Yellow', which would be more intense yellow than the un-doubled forms. The idea is one worth trying.
Further evidence on the inheritance of yellow color in rhododendrons comes from crosses in which neither parent is yellow. For example:
(a) Mr. Joseph Gable has a good hardy yellow which arose from crossing 'Boule de Neige' (white) x R. fortunei (pink).
(b) Yellows reportedly come from crosses of 'Diva', a pink (8). 'Diva' came from a cross of 'Ladybird' (pink) x R. griersonianum (scarlet). If we look into the parents of 'Ladybird', one finds R. discolor (pink) and 'Corona' (pink).
(c) One of Mr. Gable's best salmon yellows is 'Mary Belle', a cross between 'Atrier' (red) and 'Dechaem' (pink). The grandparents are 'Atrosanguineum' (red), R. griersonianum (scarlet), R. decorum (pink), and R. haematodes (red).
(d) 'Bobolink' is described as a deep yellow or apricot, yet the parents are R. discolor (pink) by R. neriiflorum (red).
From these examples it appears that recessive yellow genes or intensified genes, or both, are present in many pink and red species, particularly
R. fortunei
,
R. griersonianum
,
R. decorum
,
R. discolor
, and
R. neriiflorum
.
Most of our yellow flowered types today stem from the Neriiflorum series (
R. apodectum
,
R. scyphocalyx
, and
R. dichroanthum
); the Lacteum series (
R. lacteum
and
R. wightii
); the Thomsonii series (
R. campylocarpum
,
R. caloxanthum
,
R. litiense
;
R. telopeum
, and
R. wardii
); the Fortunei series (
R. chlorops
,
R. decorum
, and
R. vernicosum
); and the Ponticum series (
R. chrysanthum
and
R. caucasicum
). Other species reported to have intense yellow color are:
R. arizelum
and
R. codonanthum
by Bowers; (2) and
R. macabeanum
by Russell (12). The source of many of Mr. Gable's new hardy yellows is
R. vernicosum
Rock Aff. 18139, a beautiful and very hardy salmon. Seed from Rock collection 18139 appears to have been heterozygous. Mr. Gable obtained a wide variety of plant types from this introduction.
Yellow color has been known to appear suddenly as a mutant in later generations of a cross between two lily species. The flowers of
Lilium regale
and
L. leucanthum
are very similar in shape and color. They are large and funnel shaped; and the petals of both are white, with a touch of light yellow at the very base. The species were crossed in 1929, and the hybrids were intercrossed for many successive generations. None deviated in color from parental types. In 1953, a bright yellow flowered seedling appeared unexpectedly in the population; the yellow color was controlled by a single recessive gene. However, this gene was not found in either parent; consequently it was a gene hitherto unknown. (1). This is an example of the radically new types which frequently appear in later generations of species hybrids and similar wide crosses.
There is a very small group (and I must admit I am a member) that believes true dandelion yellow color in rhododendrons will probably come about by the transfer of deep yellow genes from deciduous azaleas. However, the transfer of the deep yellow color of
R. luteum
and such hybrids as 'George Reynolds', 'Bright Straw', and 'Marion Merriman', is no easy road. Crosses between non-scaly rhododendrons and deciduous azaleas are difficult; if one obtains seed, it is difficult to germinate; if the seed germinates, the progeny is usually weak; if the progeny grows well, the plants are sterile. However, we must strive to obtain good yellow azaleodendrons, and after having obtained them, we must strive to double them by the colchicine technique to obtain fertile forms that can be used in subsequent crosses. Work should also be initiated to double such yellow azaleodendrons as 'Broughtonii Aureum' and 'Glory of Littleworth.' I am fully confident that the first cross (sometime in the future) between a doubled yellow azaleodendron and a good light yellow rhododendron such as we have today, will be as significant a landmark for rhododendrons as the cross with the 'Persian Yellow' rose in 1900 was for roses.
Evergreen Azaleas:
No species with any degree of yellow color is known among the evergreen azaleas. Thus the genes for yellow color of necessity must be transferred by means of interspecific crosses. Three years ago I reported on a potential method to accomplish such crosses (7). Fortunately, we have doubled (tetraploid) forms of both evergreen azaleas and yellow-colored deciduous azaleas. Thus, the evergreen varieties, 'Wako', 'Banka', and 'Tahei' have been reported as tetraploids (6). In like manner
R. calendulaceum
is tetraploid.
Crosses between
R. calendulaceum
(female) and the three evergreen azaleas as pollen parents are easy to make. From these crosses one gets a good supply of seed and the seed germinates well. Some of the progeny are dwarfs, and none are truly vigorous. However, fully fertile hybrids are obtained.
In my crosses I used the huge flowered type of
R. calendulaceum
called 'Collossus.' Furthermore, the hybrid,
R. calendulaceum-
'Tahei', can be used as either seed parent or pollen parent in backcrossing to both parental types. At present I have growing seedlings resulting from crosses back to each original parent. In addition, crosses were successful with yellow deciduous hybrids such as 'Bright Straw'. It will be interesting to learn if yellow flowered types appear in the progenies when they reach flowering size. Of course, any plants showing yellow color will then be used to cross back again to the evergreen parents. Whether, by this method, we shall eventually end up with a bright yellow evergreen type remains to be seen. It looks very promising.
Likewise, in backcrosses to the deciduous parents, evergreen genes are incorporated into these azaleas. It will be interesting to see if the addition of evergreen genes to the deciduous types will have any effect in later generations. For example, the first generation hybrids root readily. Will this easy rooting nature he carried over into the deciduous types? At this point we can only wait and see.
In conclusion, I hope that one or more of the five points mentioned will help some rhododendron hybridizers in breeding for a purpose; or better yet, will help to put some real purpose into their rhododendron breeding.
Literature Cited
- Asen, Sam, and S. L. Emsweller, 1962, Pigments responsible for a yellow-flowered mutant hybrid from Libium regale E. H. Wilson by Lilium leucanthum Baker, Proc. Am. Soc. Hort. Sci., 81:530-534.
- Bowers, C. G., 1936, Rhododendrons and azaleas, (First Edition), MacMillan Company, 549 pp.
- Darlington, C. D., and A. P. Wylie, 1956, Chromosome atlas of flowering plants, MacMillan Company, 519 p.
- Dermen, Haig, and S. L. Emsweller, 1961, The use of colchicine in plant breeding, ARS Mimeographed Leaflet, 34-24. 10 p.
- Griggs, W. H., G. H. Vansell, and B. T. Iwakiri, 1953, The storage of hand-collected and bee-collected pollen in a home freezer, Proc. Am. Soc. Hort. Sci., 62:304-305.
- Hosoda, T., A. Moriya, and M. Sarashima, 1953, Chromosome numbers of Satsuki, Rhododendron lateritium, Genetica, 26:407409.
- Kehr, A. E., 1963 , A potential method to produce a yellow-flowered evergreen azalea, Rhododendron, 13 (3):4-5.
- Leach, D. G., 1961, Rhododendrons of the World, Charles Schribner's Sons, 544 p.
- Martin, A. C., 1963, Rimless scales on lepidote-non lepidote hybrids, Quart. Bull. Am. Rhod. Soc., 17:236-240.
- Morey, D., 1961, Observations on the inheritance of yellow pigment in roses, American Rose Annual, 1961:120-124.
- Paris, C. D., and T. J. Maney, 1944, Soleil D'Or, the progenitor of golden colored roses, Iowa Acad. of Sci., 51:247-263.
- Russell, James, 1960, Rhododendrons at Sunningdale, Privately printed, 103 p.
- Shepherd, R. E., 1954, Victory of the rose, MacMillan Company, p.177.
- Snope, A. J., and J. H. Ellison, 1963, Storage of asparagus pollen under various conditions of temperature, humidity, and pressure, Proc. Am. Soc. Hort. Sci., 83:477452.
- Visser. T., 1955, Germination and storage of pollen, Mededelinger van to Wageningen/ Nederlands 55:1-68.