Helpful Hints for Harassed Hybridists
David G. Leach, Ohio, U. S. A.
|
|
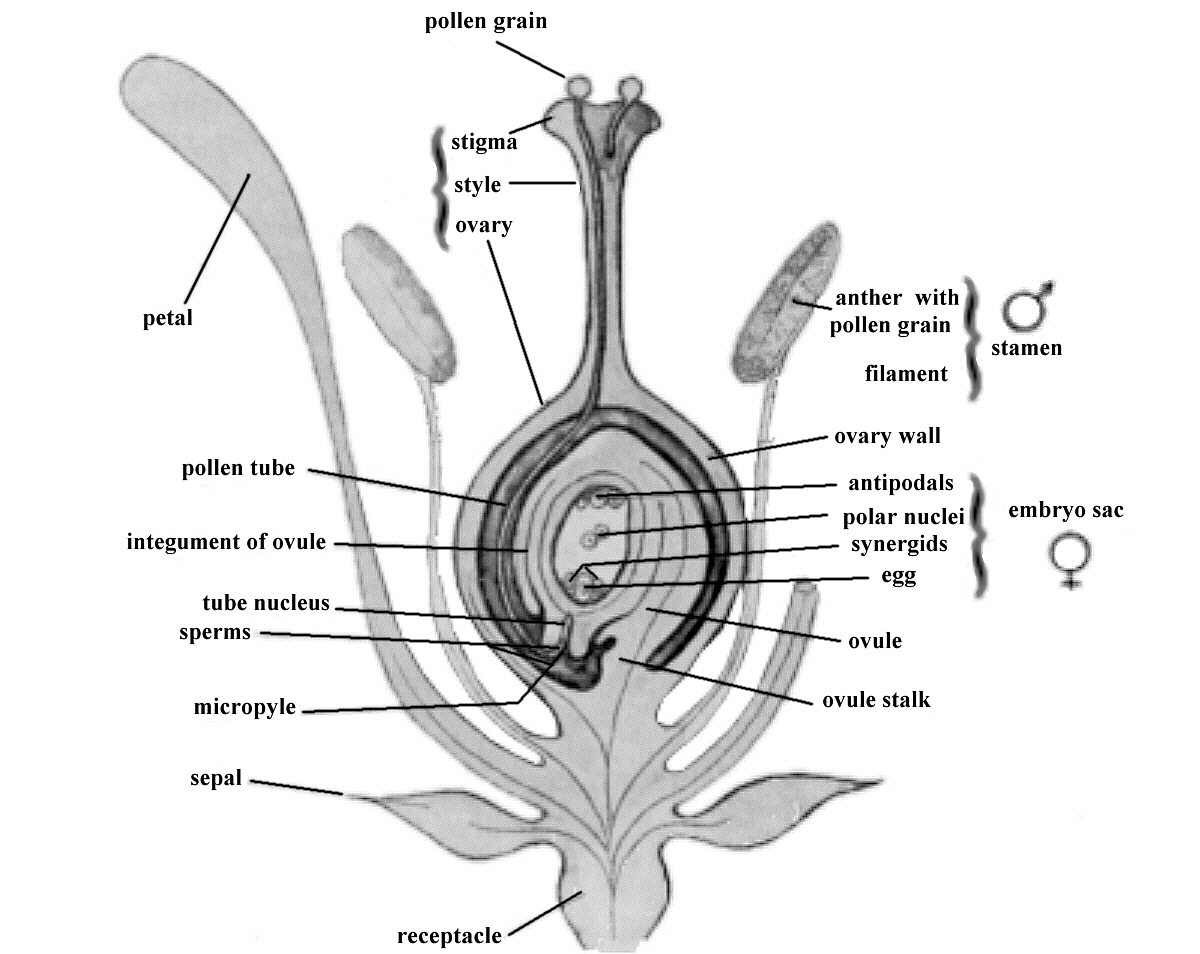
FIG. 4. Pollen, transferred from an anther to the stigma of the pistil, produces sperm cells that
fertilize egg cells in the ovary of the pistil and from the fertilized egg comes the seed. Nature accomplishes all this easily. But rhododendron lovers like to interfere, pollinating selected pistils with pollen from a selected pollen parent plant. |
When I started hybridizing rhododendrons more than 30 years ago I soon discovered that there is no instruction manual for the novice breeder. Veteran hybridists, Joseph B. Gable and Guy G. Nearing, helped me in every possible way, but they were hundreds of miles away and unavailable for advice on homely problems as they arose.
I conclude, from the questions posed to me in 1973, that the situation is not much different now, so I offer some observations which I hope will be helpful to others who are just starting down the primrose path with the bright promise of a dazzling series of new rhododendron hybrids at the end.
Nearly every beginning hybridist struggles with the manipulation of pollen so that it can be preserved for use on plants that bloom at another season. Pollen decays in storage under exactly the same conditions as do seeds - moisture and warmth. A simple way to preserve it for several weeks is to use a commercial peanut butter jar as a homemade desiccator. An inch and a half of inexpensive four-mesh anhydrous calcium chloride, ordered from a pharmacist, is covered with perforated cardboard to hold it in place. The lid should seal tightly. The calcium chloride absorbs the moisture from anthers containing the pollen, or from free pollen, so that viability is prolonged. If the pollen is to be stored more than there weeks, the desiccator should be placed in a refrigerator, ideally at 34° F. My experience with refrigerated pollen has been entirely favorable, despite published advice to the contrary. The period of preservation can be extended two months or more.
If pollen of a late blooming plant is to be saved for use on one which blooms at an earlier season it can be frozen in a deep-freeze at about 0° F. It will be still viable for making the cross the following year.
Most breeders use gelatin capsules with ribbon-like paper strips inserted which identify the pollen they contain. Small glassine coin envelopes also provide visibility of the contents. They can be labeled with a nylon-tipped pen; the envelopes are just as satisfactory as the capsules and they are far more convenient.
Anthers do not always readily yield their pollen. If the group of stamens is held in one hand and the heel of the hand is then struck sharply against the knuckle of the other, sufficient pollen may be jarred out to make a cross with a cultivar which is presumed not to produce pollen at all.
Anthers with only traces of pollen which have been in a desiccator until they are thoroughly dry can be macerated with tweezers on the palm of the hand and the entire mixture of pulverized anthers and pollen can often be used successfully to make a cross which would otherwise be impossible. Enough pollen germinates on the stigma to produce seeds, although their number is usually reduced.
Seeds of rhododendrons usually mature long before the capsules appear to be ready for harvesting, and they can be sown much sooner for accelerated growth if they are gathered early. In 1971, for example, I gathered seeds from the early blooming little blue flowered alpine,
R. fastigiatum
, on July 25th that would ordinarily not have been harvested before mid-October. They germinated perfectly. As with most new methods, a cautious trial is desirable under other conditions before a total commitment of a valuable seed lot is risked.
An exceptionally convenient identification for crosses are the British featherweight anodized aluminum Hartley Shrub Labels. They can be attached quickly at the site of the cross, and pencil parentage records on them last for years. The same label can identify the contents of the seed envelope and later the resulting seedlings in the flat, so the possibility of error is reduced.
In addition to a label of whatever sort, each flat should be marked on its side with an identifying number. The first two digits can show the year the cross was made; the second set of digits indicates the parentage as recorded in a written list of crosses to which each has been assigned a number. A felt tip marker produces waterproof ink which is very durable. Every experienced breeder has lost labels in flats to playful ground squirrels, acquisitive birds or the chubby hands of tiny tots. A second means of identification spares many an apoplectic incident.
Beginners at rhododendron breeding often pose a question which brings a harried expression to those with the best of technical backgrounds - does it make a difference, if a choice is available, which of the two parents is chosen to produce the seed?
Usually not. But then the backyard hybridist, perversely, is invariably seeking the unusual.
I have made my fair share of reciprocal crosses, as have most hybridizers, and perhaps three or four per cent, have shown differences when the same parent which produces the pollen is also used as the seed parent. The differences are not usually very large, but again amateur breeders tend to be long-shot artists. A long-shot result for me came in a winner when seedlings of
R. carolinianum
var.
album
x
R. ludlowii
produced flowers much more yellow than did the progeny of the reverse cross.
The existence of maternal inheritance, in which the seed parent exerts an undue influence upon the characteristics of the progeny, was observed with puzzled dismay by geneticists for many years. The assumption had always been that all traits are invariably controlled by the genes within the cell nuclei of the parents. Then, not too long ago, in an ingenious experiment with corn, it was demonstrated that the cytoplasm surrounding the cell nucleus of the female parent also influences the traits of the offspring, an abhorrent thought in classic Mendelian genetics. The evidence was clear, however, and the extra-nuclear determiners were called plasmagenes. They are thought to be responsible when, in reciprocal crosses, the offspring are different. The inference is that there is a long-odds mathematical advantage in choosing as the seed parent the plant which exhibits to the more marked extent the characteristics most sought in the progeny.
It is not necessary to place protective coverings over emasculated rhododendron flowers to prevent bee-carried pollen from contaminating crosses, if the corolla and stamens are removed, providing the local conditions are first tested. A few flowers should be emasculated without later fertilizing them.
If no seeds form, the breeder is reasonably safe in assuming that insects are not making natural crosses with unwanted parents, and a tedious chore can be eliminated.
In western Pennsylvania, where bumble bees visit rhododendron flowers in huge numbers almost to the exclusion of other insects, no precautions other than emasculation are needed. In northeastern Ohio, where honey bees are conspicuous in rhododendron plantings, emasculated test flowers regularly produce some seeds. Presumably the smaller, more agile honey bees roam over the receptive stigmas.
For such slow-growing seedlings as rhododendrons, milled sphagnum moss is the preferred germinating medium for all but the most sophisticated of growers. It is inexpensive, light in weight, and easily handled. The moss contains an inbuilt anti-fungicidal agent which virtually eliminates the ever-threatening scourge of damping off root rots, Phytophthora and Pythium. The United States Department of Agriculture's Research Service has evidence that sphagnum moss inhibits growth to some degree, but most old time breeders will swap this modest disadvantage for seedlings free of disease.
The most common mistake of novice breeders in pricking out seedlings is to choose those that are the strongest and sturdiest. Instead, they should be taken just as they occur in the germinating medium, whether they are weak, average or of exceptional vigor. The desired combinations of ornamental characteristics are often not associated with extraordinary vigor. They may be associated with average stature or even with a slow rate of growth.
Sooner or later small seedlings will be attacked by fungus disease or insect pests. Once established, the most common diseases are extremely difficult to control. The sensible precaution is to start spraying the seedlings when they produce their first set of true leaves in the germinating medium, and to repeat every two weeks throughout the first growing season. The best prophylaxis I have discovered is a spray solution composed of one tablespoon of 50% Captan, one-and-a half teaspoons of 50% benomyl ("Benlate") and two tablespoons of an all purpose insecticide containing 5% Sevin, 5% Meta-systox-R, and 25o Kelthane ("Isotox"), plus 15 drops of a spreader-sticker, per gallon of water. A spray solution consisting of four teaspoons of Captan and one-and-a-half teaspoons of pentachloronitrobenzene ("Terrachlor"), plus a spreader-sticker, in a gallon of water has effectively forestalled mildew on small seedlings placed in frost-free winter storage.
At the end of the first season, rhododendron seedlings which have had supplemental fluorescent light to produce a sixteen-hour growing day are about two-and-a-half times larger than those which have had a natural dawn-to-dusk day of growth following germination.
As a rule of thumb, rhododendrons respond best to fertilization at a rate about 50% of the amount recommended for other genera. The nitrogen should be available as ammonium nitrogen rather than nitrate nitrogen and to supply potassium, the sulphate is more congenial than the chloride.
It is commonplace for novices (and old hands too) to over-fertilize. At the first indication of the browning of leaf tips on seedlings, they should be flooded with water equal to about eight columnar inches to leach out the excess fertilizer salts. A crop which would otherwise be ruined can thus be saved.