Effect of Benomyl on Rooting, Ribonucleic Acid,
and Protein Content of Difficult-to-Root Rhododendron Cultivars
1
Judith Y. Flock
2
and J. J. McGuire
3
College of Resource Development
University of Rhode Island
Kingston, Rhode Island
1
Submitted in partial fulfillment for the M.S. degree. Supported in part by a grant from the American Rhododendron Society.
2
Graduate Assistant
3
Professor of Plant and Soil Science
Rhode Island Agricultural Experiment Station Contribution No. 1746
Many plant growth regulators, including such synthetic auxins as indolebutyric acid (IBA), are effective in initiating root formation in many plant species (2, 14). The response to auxin is not universal, however, and cuttings of some difficult-to-root species still root poorly after auxin treatment. Cytokinins are endogenous plant growth regulators which are involved in several plant processes. Several investigators (3,6) have reported that the endogenous balance of auxin to cytokinin in the plant tissue determines organ regeneration, i.e., root or bud formation. Cytokinins affect the protein and nucleic acid (RNA) content of detached plant organs (9). Further, cytokinin-treated portions of tobacco leaves exhibit increased protein and RNA synthesis whereas control portions exhibit a prompt and rapid decline in protein content (16).
The systemic fungicide methyl-1-(butylcarbomoyl-2-benzimidazole carbamate) (benomyl, BEN) has demonstrated cytokinin activity (10, 12, 13). Hormone/ fungicide combinations of BEN with various auxins have also been found to improve the rooting of some cuttings (1,5,1 1). McGuire and Vallone (5) reported that the IBA/BEN combination was most effective in stimulating the rooting of difficult-to-root rhododendron cultivars while there was no significant effect on easy-to-root rhododendron cultivars.
These experiments were designed to study the effect of BEN on the rooting of difficult-to-root rhododendrons when used with an auxin application. If BEN acts as a cytokinin in the rooting response, then it may affect the RNA and protein content of the tissue. Therefore, the affect of various treatments as well as the time elapsed between removal of the cuttings and chemical analysis of the tissue were studied.
Materials and Methods
A. Rooting Study. A rooting study was performed in 1975 on several difficult-to-root cultivars to determine the effects of BEN and N6benzyladenine (BA) applied either alone or in combination with the auxin IBA. Cuttings of the rhododendron cultivars 'C. S. Sargent', 'Francesca', 'Dr. H. C. Dresselhuys', and 'Goldsworth Yellow' were used.
The treatments were prepared as talc formulations. Whenever I BA was used the concentration was constant at 45,000 ppm. The treatments were: IBA applied alone or in combination with 50,000 ppm BEN or 10 ppm BA, BEN alone at 50,000 ppm, BA alone at 10 ppm, and a control. The propagation medium consisted of sphagnum peat moss and horticultural perlite in a 1:1 (v/v) ratio. The rooting response was measured by use of an index based on rootball size. Rootballs less than 25mm in diameter received an index number of 1, those between 25 and and 49mm in diameter 2, those between 50 and 74mm in diameter 3, those between 75 and 99mm in diameter 4, and those 100mm or greater 5. Sample average indices were compared within each cultivar to determine response to treatment.
B. Protein and RNA determinations. 'C. S. Sargent' was used to determine the effect of the treatments on the protein and RNA content of cuttings during rooting. The treatments were the same as described above. Three cuttings per treatment were removed from the rooting medium at 2, 7, 11 and 22 days after treatment. The basal 30mm of bark of each cutting including a thin sliver of xylem were removed. Samples were freeze-dried and ground in a Wiley Mill. Forty mg samples were homogenized in 2 ml of buffer (pH 7.6) containing 2.5% polyvinyl polypyrollidone to precipitate interfering phenolic compounds, centrifuged, and the solid residue discarded. Subsequently, nucleic acids and protein were separated in the supernatant by protein precipitation with an equal volume of cold 20% trichloroacetic acid. Protein was dissolved in 0.1 N sodium hydroxide for analysis. Aliquots of the nucleic acid and protein fractions were used for RNA and protein determinations. Ribose from RNA was determined by using orcinol and protein by using the Folin-Ciocalteu reagent for detecting phenolic hydroxyl groups present in proteins. A standard curve for RNA concentration was constructed using yeast-RNA and a standard curve for protein constructed using bovine serum albumin. The presence of RNA was indicated by the production of green color, the intensity of which indicated concentration. The color was determined at 650 nm using a spectrophotometer. The presence of protein was indicated by the production of blue color, the intensity of which indicated concentration. The color was determined at 660 nm on the spectrophotometer. RNA and protein contents of the cuttings were compared to determine the effect of the treatments as related to the number of days between treatment and removal of cuttings for chemical analysis.
C. Cytokinin bioassay. A Raphanus (cv. "Long Scarlet Short Top") cotyledon bioassay was performed (4) to test for cytokinin activity of BEN: benzimidazole (BIA), the parent compound of BEN; and BA at 0.01, 0.1, 1.0, 5.0, 10.0, 50.0, 150.0 and 500.0 ppm. A 6-furfurylaminopurine (Kinetin) standard was used for comparison. BEN was prepared as a suspension while BIA and BA were prepared as solutions. Three mls of each compound at each concentration were added to petri dishes containing 8 cotyledons. The initial fresh weights and terminal fresh weights after 3 days at 26° C + 4° C under continuous fluorescent lighting were measured. Results were expressed as percent increase or decrease in fresh weight. Cytokinin activity was indicated by an increase in fresh weight due to cotyledon enlargement.
Results and Discussion
| Table 1. Effect of IBA, BEN and BA on rooting of rhododendron in 1975. z | ||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| z Mean separation in columns by Duncan's Multiple Range Test, 5% level. | ||||||||||||||||||||||||||||||||||||||||||||||
|
y
Root ball index: mean root ball diameter:
(in mm) |
0 | 1-24 | 25-49 | 50-74 | 75-99 | 99 | ||||||||||||||||||||||||||||||||||||||||
|
index no. |
0 | 1 | 2 | 3 | 4 | 5 | ||||||||||||||||||||||||||||||||||||||||
| x IBA concentration constant 45-000 ppm. | ||||||||||||||||||||||||||||||||||||||||||||||
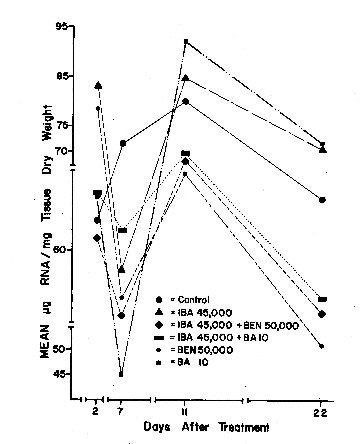
Rooting. 'Goldsworth Yellow' and 'Francesca' were used for the rooting study (Table 1) because they are extremely difficult-to-root and are propagated commercially by grafting. 'Goldsworth Yellow' rooted poorly and although no significant differences were observed between treatments, the IBA plus BEN 50,000 ppm did show promise by producing the greatest amount of rooting. The same trend was observed in 'Francesca' with the IBA plus BEN 50,000 ppm producing the greatest amount of rooting, followed by I BA plus BA 10 ppm. There appeared to be a lot of variation in 'Francesca' due to factors other than treatment and therefore no significant differences were observed between the IBA containing treatments. In 'Dr. H. C. Dresselhuys' and 'C. S. Sargent' (Table 1) the greatest amount of rooting occurred with I BA plus BEN 50,000 ppm. The IBA plus BA 10 ppm also caused a substantial amount of rooting. BA and BEN alone had little or no effect on rooting when compared to the control treatment. With respect to the theory of the role of the cytokinin/auxin ratio or balance in regeneration (6), perhaps endogenous auxin are present in the cuttings of these difficult-to-root rhododendrons in sufficient quantity for rooting to occur but cytokinins are limiting. It is possible that BEN and/or BA may have stimulated rooting by acting as a cytokinin and shifting the auxin/cytokinin ratio to a more favorable balance for rooting. It may be possible that the correct combination of cytokinin/auxin was not reached in some cases, i.e. 'Francesca' and 'Goldsworth Yellow' and therefore significant differences were not observed although the trend was observed, or perhaps the lack of rooting is due to other factors such as the presence of inhibitors.
Protein and RNA determinations.
|
Table 2. Mean RNA content of 'C.S. Sargent' stem tissue at 2, 7, 11 and
22 days after treatment z |
|
| Sampling date |
Mean ug RNA/mg tissue (dry wt) |
| (days after treatment) | |
| 2 | 65.9b |
| 7 | 57.3c |
| 1 1 | 70.8a |
| 22 | 61.2bc |
| z Mean separation in columns by Duncan's Multiple Range Test, 5% level. | |
|
|
There were no significant differences among the treatment and control means of the RNA analyses as presented in Figure 1. If the effect of BEN and BA, in combination with IBA, is due to cytokinin activity, they may be affecting some process other than RNA synthesis. There were significant differences, however, among sampling dates (Table 2), i.e., the amount of time elapsed between treatment and analysis of the tissue had a significant effect. There appeared to be 2 phases in the pattern of RNA content over time (fig. 1). The first phase was evident 7 days after cuttings had been removed from the stock plants and placed in the rooting media wherein the RNA level declined to the lowest level in the analysis. The second phase occurred between the seventh and eleventh day after treatment when the RNA level appeared to peak. In 1939, Went (15) concluded that there were 2 phases leading to root formation. In 1969, Mitsuhashi, et al (7.8) reported that there were 2 phases leading to adventitious root formation in Azukia (Phaseolus). The first phase was the production of root initials and the second phase was rootlet development from the primordia. They characterized the second phase as requiring increased protein and nucleic acid synthesis due to vigorous cell division. The occurrence of 2 phases in the RNA content of the cuttings may be evidence for the possibility of a delayed treatment effect. It may be more effective to treat a cutting to induce rooting after some time has passed after removal from the stock plant rather than immediately after the cuttings have been taken. Perhaps the cutting's ability to respond to treatment varies between the 2 phases.
| Table 3. Percent Increase in Fresh Weight of Raphanus Cotyledons Floated for 64 Hours on Different Concentrations of KIN, BEN, BIA and BA | ||||||||
| Concentration (ppm) | ||||||||
| Treatment | 0.01 | 0.1 | 1.0 | 5.0 | 10.0 | 50.0 | 150.0 | 500.0 |
| KIN | 121.5z | 146.7 | 192.6 | 140.9 | -69.5 | -91.4 | -93.8 | -95.4 |
| BEN | 110.8 | 124.8 | 131.1 | 175.5 | 228.1 | 286.3 | 302.6 | 241.4 |
| BIA | 114.6 | 93.2 | 47.0 | -17.1 | -46.5 | -69.7 | -73.8 | -99.5 |
| BA | 25.8 | -33.0 | -62.7 | -68.6 | -73.5 | -79.8 | -97.0 | -88.6 |
| z Weights adjusted by subtracting the weight of the water uptake occurring in control treatment. | ||||||||
There were no significant differences among the sampling dates of the protein contents. Very little change was observed in protein contents due to treatments. Protein changes may be expected to lag behind RNA changes since RNA synthesis is a prerequisite for protein synthesis. Perhaps if the experiment had been continued for a longer period of time, a change would have eventually been observed reflecting the RNA pattern.
Bioassay. Cotyledons treated with BEN showed an increase in fresh weight up to 150 ppm and at 500.0 ppm it was inhibitory (Table 3). BEN does exhibit some cytokinin activity as demonstrated by these results. Most cytokinins are physiologically active at low levels of 1.0 ppm or less and therefore the increase in fresh weight at 10.0 ppm BEN and above seems to be well beyond that which could be attributed to cytokinin actions and may be due to some other activity. BEN's poor solubility may cause poor entrance into the plant and perhaps its weak activity compared to the kinetin standard. The peaks for BIA and BA occurred at the lowest concentration tested. All higher concentrations were inhibitory. Both compounds may be more effective at a lower concentration than was tested.
Summary
In this study, rooting response of 'Dr. H. C. Dresselhuys' and 'C. S. Sargent' was improved by using a combined indolebutyric acid and benlate treatment. The same trend was noted for 'Francesca' and to a lesser degree for 'Goldworth Yellow.' It was theorized that hard-to-root rhododendrons may contain auxins in sufficient quantity for rooting to occur but cytokinins might be limiting. Benlate in lower concentrations (at about 150 ppm) acts as a cytokinin and hence for the four hard-to-root rhododendrons used in these experiments, provided a more favorable auxin-cytonkinin balance for rooting.
From the results obtained in these experiments it was suggested that perhaps rooting of hard-to-root rhododendrons may be enhanced if the cuttings were treated with IBA or IBA-KIN 1 to 2 weeks after being placed in the rooting media.
High concentrations of benlate appeared to be inhibitory.
Literature Cited
1. Fiorino, P., J. N. Cummins, and J. Gilpatrick, 1969, Increased production of rooted Prunus besseyi (Bailey) softwood cuttings with preplanting soak in benomyl, Proc. Inter. Plant Prop. Soc., 19:320-329.
2. Hartmann, H. T. and D. E. Kester, 1968, Plant propagation: principles and practices, 3rd edition. Prentice-Hall, Inc., N.J. 702 pp.
3. Heide, O. M., 1965, Interaction of temperature, auxin, and kinins in the regeneration ability of Begonia leaf cuttings, Physiol. Plant, 18:891-920.
4. Letham, D. S., 1968, A new cYtokinin bioassay and the naturally occurring cytokinin complex, Pages 19-31 in Wightman and Setterfield, eds. Biochemistry and physiology of plant growth substances.
5. McGuire, J. J. and V. H. Vallone, 1971, In-teraction of 3-Indolebutyric acid and benomyl in promoting root initiation in stem cuttings of woody ornamental plants, Proc. Inter. Plant. Prop. Soc., 21:374-380.
6. Miller, C. O. and F. C. Skoog, 1953, Chemical control of bud formation in tobacco stem segments, Amer. J. Bot., 40:768-773.
7. Mitsuhashi, M., H. Shibaoka, and M. Shimokoriyama, 1969, a. Portulal: a rooting promoting substance in Portulaca leaves. Plant and Cell Physiol. 10:715-723.
8. Mitsuhashi, M., H. Shibaoka, and M. Shimo Koriyama, 1969, b. Morphological and physiological characterization of IAA-sensitive and IAA-less sensitive phases in rooting of Azukia cuttings, Plant and Cell Physio, 10:867-874.
9. Richmond, A. E. and A. Lang, 1957, Effect of kinetin on protein content and survival of detached Xanthium leaves, Science, 125:650-651.
10. Skene, K. G. M., 1972, Cytokinin-like properties of the systemic fungicide benomyl, J. Hort. Sci., 47:179-182.
11. Thiegles, B. A. and H. A .J. Hoitink, 1972, Fungicides and rooting of Eastern white pine cuttings, For. Sci. 18:54-55.
12. Thomas, T. H., 1973, Growth regulatory effect of three benzimidazole fungicides on the germination of celery (Apium gravedens) seeds, Ann. App/. Biol., 76.233-238.
13. Thomas, T. H., 1974, Investigations into the cytokinin-like properties of benzimidazolederived fungicides, Ann. Appl. Biol., 76:237-241.
14. Weaver, R.J., 1972, Plant growth substances in agriculture, W. H. Freeman, and Co., Calf. 594pp.
15. Went, F. W., 1939, The dual effect of auxin on root formation, Amer. J. Bot., 26:24-29.
16. Wollgiehn, R., 1961, Untersuchungen uber den einflub des kinetins auf den Nucleinsaureund proteinstoff- Wechsel isolierter Blatter, Flora, 151:411-437. (English Summary).