Mycorrhizae and Rhododendron
By Larry Englander, Ph.D.
University of Rhode Island, Kingston, Rhode Island
Reprinted from The Rosebay
Rosebay Note: Chapter member Larry Englander, is an assistant professor in the Department of Plant Pathology-Entomology at the University of Rhode Island. He has worked with Phytophthora diseases for a number of years, studying a root rot of Chamaecyparis lawsoniana for his doctoral thesis at Oregon State University. Several years ago, Dr. Englander became interested in mycorrhizae of rhododendron as a possible factor in root rot suppression. The results of his studies, only briefly described-in his article, are very exciting - to say the least. It is both interesting to note and gratifying to learn that his original work was sponsored by a grant from our own A.R.S. Research Foundation. The latter led to a further grant from the Agricultural Experiment Station, U. R. I. and recently he presented a paper on his work at the Fourth North American Conference on Mycorrhizae. Those of you who wonder where your Research Foundation dollars go, and what they do - look no further!
Plant roots serve a number of important functions, not the least of which are the absorption of minerals and water, and the storage of carbohydrates manufactured by the foliage. Roots do a creditable job of supporting healthy, thriving plants, but are inefficient in one respect - they leak. Often a large variety of harmless microorganisms are attracted into the root system's zone of influence (the rhizosphere) where they join a "soup line", eagerly awaiting unintentional handouts in the form of exudates by the plant roots. Through evolution, some fungi gave up the humble, patient ways of their companions and rather than waiting outside for a handout, instead take their sustenance by force, penetrating and invading the root.
In this latter group are the pathogenic fungi, such as Phytophthora root rot fungus, whose tiny swimming spores use rhododendron root exudates as a beacon to direct them to the root which they proceed to infect and destroy. Obviously, only the pathogen derives benefit from this interaction. But there is another relationship that has developed between certain fungi and roots, in which the fungus may penetrate root cells but not kill them. In fact, both the root and the fungus derive benefits from this association, called a mycorrhiza (see Ted Van Veen's article in the Fall, 1978, Quarterly Bulletin, A.R.S.)
A peculiarity of roots of rhododendrons and other ericaceous plants is that they do not have root hairs, which are minute projections of the epidermis that compensate for their small size by occurring in great abundance, increasing root surface area and the number of sites with which the roots can "mine" for minerals and water. The absence of root hairs in rhododendrons may be partially offset by the characteristic dense fibrous root system, but substituting for root hairs on many rhododendrons throughout the world are the microscopic threads of fungi in a mycorrhizal relationship.
Although the classification of kinds of mycorrhizae is not stable, with new or intermediate types of relationships periodically being discovered, the rhododendron mycorrhizae are generally classified as ericoid endomycorrhizae, a group characterized by fungus penetration into cells on the root surface. Looking through the microscope at a young rhododendron root, one finds up to 80% of the surface cells have been penetrated by a fungus, each cell containing a "knot" of fine fungal filaments, called hyphae. Remarkably, these invaded root cells are not destroyed, but continue to function. Outside these cells there is a thin weft of hyphae acting like a microscopic root system, weaving together the plant roots and soil particles some distance away. By investing part of its hyphae inside the root cell, the fungus benefits by obtaining essential carbohydrates with out having to compete with the myriad of hungry microorganisms in the soil. The hyphae in the soil absorb water and minerals, some of which are trans-located to the "knot" of hyphae inside the root cell. In some manner not yet fully understood, the root obtains from the fungus a share of these materials for its own use. This increases the plants' access to minerals, especially important when these materials are present in the soil in limited quantities.
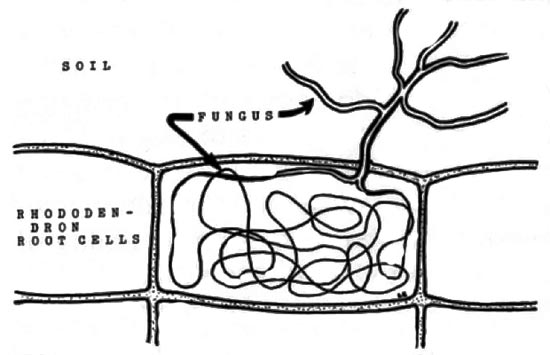
|
|
Rhododendron root cell. Note mycorrhizal fungus hyphae, "knot",
within cell and extending into the soil. Drawing by Dr. Larry Englander |
Our knowledge about mycorrhizae on ericaceous plants has been greatly increased by the elaborate research of a group in England, directed by Dr. D. J. Read. They were able to culture a fungus which formed mycorrhizae with cranberry, heather and rhododendron, and they demonstrated under laboratory conditions that this fungus, Pezizella ericae stimulated the growth of cranberry and heather seedlings, and increased their phosphorus and nitrogen content when grown where these minerals were in a form not readily available to plant roots.
Several years ago, I began studying another fungus as a potential mycorrhiza on ericaceous plants. This study was indicated by the observations detailed below, and was encouraged by a grant from the A.R.S. Research Foundation. Each year we found large numbers of fruiting bodies of a particular fungus on the ground beneath rhododendrons and related plants. The sites, thus far only in Rhode Island and adjacent states, varied considerably, from those exposed to full sun and wind on large expanses of flat terrain, to woodland settings where shade, wind protection and natural mulch provided a more moderate environment for the plants. Soil types varied also, but generally were of low fertility. It was interesting to find the fungus fruiting beneath rhododendrons in home gardens, but there was indeed a spectacular sight to behold when visiting certain wholesale nurseries which grow large tracts of rhododendrons. There I found hundreds of these small fruiting bodies under nearly every ericaceous plant, and not a single one beneath any other plant, even when both types were interspersed in the same rows. The specificity of this fungus/plant relationship is striking. A partial list of rhododendron cultivars and species with which the fungus has been found associated appears in Table I. Other ericaceous plants found with the fungus include
Kalmia latifolia
,
Pieris floribunda
and
japonica
, and
Leucothoe
.
| Table 1. Rhododendron species and cultivars. | |
| 'Boule de Neige' | 'Lee's Dark Purple' |
| calendulaceum | mollis |
| 'Caractacus' | 'Mrs. C.S. Sargent' |
| catawbiense album | 'Mrs. P. den Ouden' |
| 'Chionoides' | maximum |
| 'Cunningham's White' | mucronulatum |
| 'Dr. Dresselhuys' | nudiflorum |
| 'English Roseum' | 'Nova Zembla' |
| 'Francesca' | 'PJM' |
| 'Goldsworth Yellow' | 'Rosebud' |
| 'Ignatius Sargent' | schlippenbachii |
| kaempferi | vaseyi |

|
|
Fruiting body of mycorrhizal fungus, Clavaria
Photo by Larry Englander |
The fungus fruiting bodies look like slender cream-colored stalks, generally one to two inches tall, and these occur either singly or in clusters. They first appear in the autumn, at about the time of the first frost, and continue to emerge until the deep freeze sets in. Each fruiting body produces millions of microscopic spores, which are disseminated by wind to other areas, presumably to initiate mycorrhizae, if they fortuitously land near the root of an ericaceous plant. Of course, these readily visible fruiting bodies comprise only the reproductive part of the fungus, the remainder being a vast network of microscopic hyphae in the soil and plant roots. The fungus has been identified as a
Clavaria
species, and prior to our work had not been reported as associated with mycorrhizal rhododendron roots in the United States. It was reported in Australia in 1973, on container grown azaleas.
Finding fungus fruiting bodies constantly associated with certain types of plants is not sufficient evidence that the same fungus is the one forming mycorrhizae on the roots. It was very difficult to trace hyphae connecting the
Clavaria
fruiting body with the root due to the microscopic size of these fragile strands and the inability to see through soil particles. Another approach to verifying the mycorrhiza-forming ability of a fungus is to "synthesize" a mycorrhiza in the laboratory by combining a pure culture of the suspected fungus with an aseptically grown plant. Thus, when mycorrhizal roots develop, this constitutes evidence that the intentionally applied fungus was responsible. Since we haven't yet found a method for growing the
Clavaria
fungus in the laboratory, we used another method to determine whether there was a connection between its fruiting bodies and the roots - applying a radioactive isotope to one side of the plant/fungus pathway and monitoring its passage across to the other side. We utilized the phenomena that plant sugars are passed to mycorrhizal fungi, and phosphate may be transferred from fungus to plant roots.
In the first part of the study, radioactive carbon dioxide (14c) was injected into plastic bags covering shoots of six year old plants of
Pieris Japonica
and R. 'English Roseum' growing in the field at the University of Rhode Island research farm.
Clavaria
fruiting bodies under these "labeled" plants were sampled after various periods of time during which the 14c was assimilated by the leaves during photosynthesis and converted to sugars which were trans-located to the roots.
The mycorrhizal hyphae in these roots normally take a share of these sugars, some of which contain 14c. If the
Clavaria
fruiting bodies were part of the mycorrhizal fungus, and therefore connected to the roots, then these fruiting bodies should become radioactive as they acquire sugars, through their hyphae, from roots of the "labeled" plants.
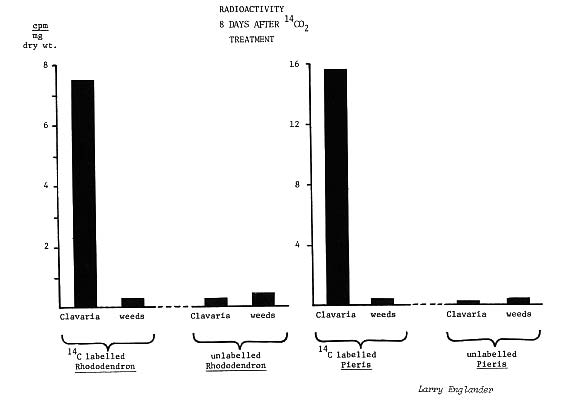
Indeed, high levels of radioactivity were measured in fruiting bodies taken from beneath the 14c "labeled"
Pieris
and rhododendrons (25-fold and 121-fold increase, respectively), while weeds under these same plants, and both
Clavaria
fruiting bodies and weeds under untreated plants, had only background (environmental) levels of radioactivity.
|
In another series of tests, radioactive phosphate (32p) was applied to
Clavaria
fruiting bodies under two year old field grown rhododendron liners ('English Roseum') which had been transplanted to the greenhouse. Subsequently we assayed root samples from at least 23 mm below the fruiting bodies, and also roots from a point midway between the plant stem and the treated fruiting bodies, In most samples, roots were considerably more radioactive (approx. 20 to 600-fold increase) than control roots from plants with fruiting bodies that had not been treated with 32p. Additional tests showed that when 32p was applied directly to the soil surface rather than the fungus, there was no radioactivity increase of roots 12 mm or more below the surface, nor in roots at a point midway between the plant stem and the treated soil.
Thus, there appears to be an efficient two-way pipeline between mycorrhizal rhododendrons and
Clavaria
fruiting bodies. This supports the hypothesis that
Clavaria
is mycorrhizal on rhododendrons. Also significant is the ability of this fungus to supply roots with phosphorus. Roots often have only limited access to phosphate in the soil, since the insolubility of this mineral hinders its redistribution in soil water. Whether mycorrhizal roots obtain more phosphate due to the "mining" of more sites in the soil by fungus hyphae, or through the ability of the fungus to dissolve and obtain forms of phosphorus which plant roots cannot, is controversial.
Currently we are using electron microscopy to examine the effect on rhododendron root cells of mycorrhizal fungi, and found that several different fungi are probably capable of forming mycorrhizae. Other studies underway include the effect of mycorrhizae on rhododendron seedling growth rates, the inhibition of ericoid mycorrhizae by fungicides, the influence of plant culture medium composition and sterilization on time and degree of mycorrhizal establishment, and physiological investigations on nutrient uptake by rhododendrons with and without mycorrhizae. We hope to continue studying the poorly understood ericoid mycorrhizae to determine in which processes and to what degree this association is beneficial to rhododendrons, which micro-organisms are most appropriate, and whether or how our help may be required for initiating and encouraging mycorrhizae of ericaceous plants.
REFERENCES
:
Englander, L. and R. J. Hull, 1980, Reciprocal transfer of nutrients between ericaceous plants and a Clavaria sp., New Phytol., 84 (4): (in press).
Read, D.J. and D. P. Stribley, 1975, Some mycological aspects of the biology of mycorrhizae in the Ericaceae., pages 105-117 in: F. L. Sanders, B. Mosse and P. B. Tinker, eds. Endomycorrhizas, Academic Press, NY, 626 p.
Seviour, R. J., R. R. Willing and G. A. Chilvers, 1973, Basidiocarps associated with ericoid mycorrhizas, New Phytol., 72: 381-385.
Stribley, D. P. and D. J. Read, 1975, Some nutritional aspects of the biology of ericaceous mycorrhizas., pages 195-207 in: F. L. Sanders, B. Mosse and P. B. Tinker, eds. Endomycorrhizas, Academic Press, NY, 626 p.