Some Notes on Reproduction in R. maximum and R. minus
D. C. Purdy, Huntington, N.Y.
1. Introduction
Rhododendron maximum
has been used in hybridization because of its hardiness and adaptability to eastern United States growing conditions. Leach (1) lists 49 hybrids containing
R. maximum
. A compilation of more recent data brings the total of known hybrids to 64.
Rhododendron maximum
has been shown to be a difficult plant to hybridize with (2). Apomixis is a common problem.
Rhododendron minus
, another eastern United States species, is not commonly used in hybridization, being overshadowed by its close relative,
Rhododendron carolinianum
. Leach (1) lists only three minus hybrids. I have done some hybridization using
R. minus
, and have found frequent apomixis and failure to produce seed.
Recently, some taxonomists have combined
R. minus
and
R. carolinianum
into one species,
R. minus
(A), while others (5) still recognize two species. These notes will use the older nomenclature to distinguish between what some now regard as two fractions of the same species.
This investigation was undertaken to clarify the reasons for frequent apomixis with these two species and to explore the implications of the apomixis for reproduction. A recent paper (3) regarding
Gaultheria procumbens
, another ericaceous plant from the eastern United States, suggested that that species used self pollination as a normal reproduction mechanism. The possibility existed that the subject species might follow suit.
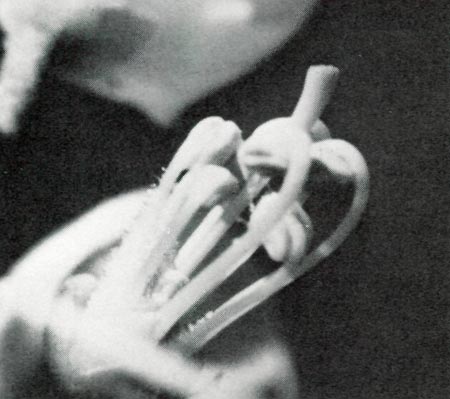
|
|
Figure 1
photo by D. C. Purdy |
2. Discussion of
R. minus
Florets of
R. minus
were dissected when in bud. It was found that the stamens and pistil were in a common compartment, unlike some varieties (e.g.
R. yakushimanum
x 'Midsummer'), where the pistil is in a separate compartment formed by one of the petals. The stamens expand first and the pistil then expands through and beyond them (photo 1). At this stage the pistil is not receptive but ample pollen is available. The pistil was not sticky, as it was later. Also, attempts to hand pollinate were unsuccessful until four days later. The flower opened in one day. When the floret opened, the pistil drooped, assuming a position below the stamens. The droop makes hand pollination difficult, but appears to present no difficulty to bee pollination. This feature may reduce self pollination, since any pollen which is deposited before the stigma is receptive has an opportunity to drop off. The pistil on
R. carolinianum
does not droop as much, making hand pollination easier.
To determine whether self pollination was a normal process, one plant of
R. minus
was enclosed in a screen cage to exclude insects (photo 2). The cage was positioned prior to bloom and removed in October. No seed pods developed, so it would appear that self pollination is not a common mode of reproduction. Neighboring plants of
R. minus
which were not enclosed did produce normal seed.

|
|
Figure 2
photo by D. C. Purdy |
3. Discussion of
R. maximum
The dissection of
R. maximum
flowers gave results similar to
R. minus
. In the flowers we dissected, the pistil was always longer than the stamens, but in close proximity (photo 3). The dissections generally confirmed earlier observations by Ring and Goodrich (6). Again, the stigma was not receptive until after the flower opened. At the latter stage, the stigma was in a favourable position for fertilization by insects and humans.
A plant of
R. maximum
with five flower buds was placed in an insect proof cage. It produced three seed pods from one truss, each containing a small amount of viable seed. The viability of the seed was proved by sprouting. Neighboring exposed plants produced approximately eleven seed pods per truss, each containing more seed. It therefore appears that seed production for the enclosed plant was one or two percent of what it would otherwise have been.

|
|
Figure 3
photo by D. C. Purdy |
4. Conclusions
When crossing with either
R. minus
or
R. maximum
, self pollination can be prevented only by early and careful emasculation of the flower. Care must be taken to avoid getting pollen on the stigma where it might later cause fertilization. In
R. minus
, fertilization will always be difficult because the stigma faces downwards when receptive.
In nature, both plants are normally insect pollinated. In our experiments, only
R. maximum
was self pollinated, and then only to a minor extent.
References
1. Leach, David G. 1961 Rhododendrons of the World
2 Leach, David G. 1965 The Rosebay Rhododendron: Historical Oddities, Unusual Forms, Its Value as a Parent. Quarterly Bulletin of the American Rhododendron Society 19 pp. 66-74
3. Mirick, Sally and Quinn, James A. 1981 Some Observations on the Reproductive Biology of Gaultheria Procumbens (Ericaceae). American Journal of Botany 68 pp. 1298-1305
4. Cullen, J. 1980 A Revision of Rhododendron. Notes from the Royal Botanic Garden Edinburgh 39 no. 1
5. Davidian, H. H. 1982 Rhododendron Species vol. 1
6. Ring, George W. and Goodrich, Raymond H. 1972 Getting the Max out of R. Maximum or "Apomixis, Anyone?" Quarterly Bulletin of the American Rhododendron Society 26 pp. 263-265