Short Term Pollen Storage Of Two Rhododendron simsii Cultivars
Mark P. Widrlechner, Ph.D.
North Central Regional Plant Introduction Station
Iowa State University, Ames, Iowa
Introduction
Rhododendron breeders work with a genus that collectively has a broad flowering season. However, many individual species or cultivars flower for much shorter periods. To make many desirable crosses, it becomes necessary for the breeder to store pollen. Existing literature offers only brief recommendations on storage conditions in reports by Bowers (1932), Lee (1958), and Schroeder and Bump (1982) and a single, more detailed report by Visser (1955) that few breeders have available.
This study outlines the relative value of a range of storage conditions for short-term (two weeks or less) pollen storage of two cultivars of evergreen azaleas. Long-term storage is generally done successfully by using variations of the desiccator-freezing method, as described by Schroeder and Bump (1982) and Visser (1955).
Materials and Methods
Plants of
Rhododendron simsii
Planch cultivars, 'Alaska', a Rutherford hybrid, and 'Variegated Dogwood' (Plant Patent 4455), were purchased from a local florist. The plants were about to flower heavily, with many buds just beginning to open.
Anthers were collected from the flowers on 5 January 1986, just as they opened. Two groups of six, unsealed, 2-dram glass vials, with each vial holding three anthers of a single cultivar, were placed in one of six storage conditions. Sample vials were removed from storage at 1, 2, 3, 4, 7, and 10 or 14 days after collection and were allowed one hour to equilibrate to laboratory conditions. Pollen viability was then calculated by using the 3-(4, 5-dimethylthia-zolyl-2)-2, 5-diphenyl tetrazolium bromide (MTT) vital staining method, as outlined by Hecker (1963), which has been shown to be an accurate indicator of
in vivo
pollen germination in deciduous azaleas (Widrlechner etal., 1983). From each of the three anthers, 200 micro-spores were scored after staining, making a total sample of 600 micro-spores per treatment for each cultivar.
As a control, on each day of the experiment, three anthers were sampled from newly opened flowers from the donor plants. Based on these counts, a mean staining proportion was calculated for each cultivar. The test results are then presented graphically with sample staining shown as a proportion of the control means. The six test conditions for pollen storage were:
Condition A: Anthers were stored at room temperature (17-24°C) with no humidity adjustment (32-42% relative humidity).
Condition B: Anthers were stored at room temperature in a large glass desiccator with anhydrous CaSO4 as a drying agent.
Condition C: Anthers were stored in an incubator at 20°C for 16 hours per day and 30°C for 8 hours per day with 80-100% relative humidity.
Condition D: Anthers were stored in a refrigerator at 4°C.
Condition E: Anthers were stored in a freezer at -18°C.
Condition F: Anthers were first placed in a large glass desiccator, as in Condition B, for 18 hours; then they were moved for storage in the freezer along with samples of Condition E.
Results and Discussion
Based on a total of 4200 micro-spores compiled from measurements taken on seven days, the mean proportion of mico-spores of 'Alaska' stained by MTT is 0.356 ± 0.078 (±1 S.D.). For 'Variegated Dogwood', the proportion is 0.408 ± 0.058. These figures are considerably lower than found in the majority of deciduous azalea cultivars, as surveyed by Widrlechner and Pellett (1983), but are consistent enough within each cultivar to use for this experiment. To date, no comparable survey of pollen viability of evergreen azalea cultivars has been published.
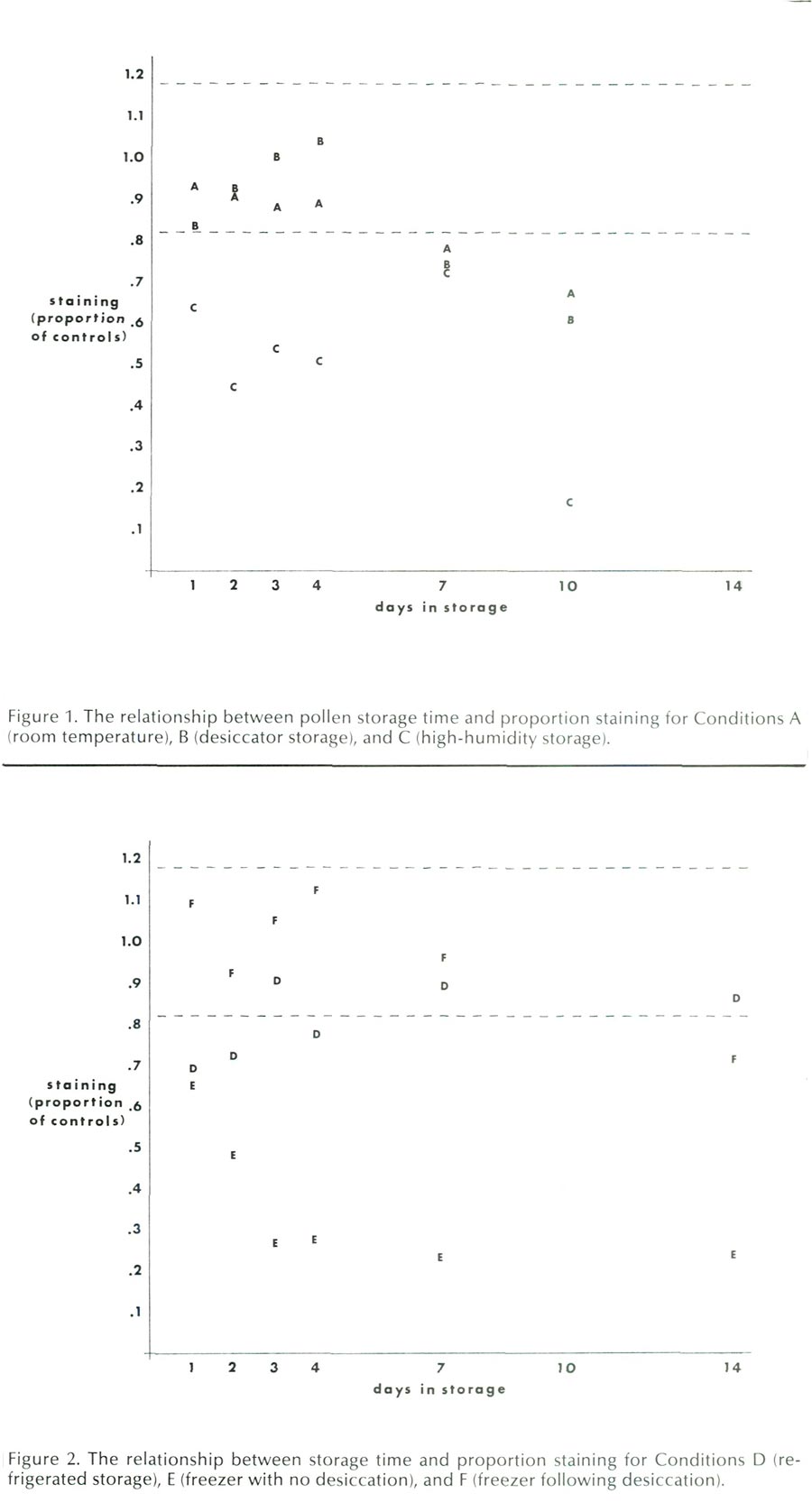
The results of the room temperature storage conditions, Conditions A, B, and C, are presented in Figure 1. Figure 1 contrasts days in storage with staining, as expressed as a proportion of the mean of the control samples for both cultivars. The letters indicate the mean values of each condition on a given day for the two cultivars, and the dotted lines indicate the proportion of staining found at ± 1 S.D. from the control mean. If storage treatments did not affect pollen viability, approximately two-thirds of the data points would fall within the area demarcated by the dotted lines. This was not the case.
Condition A, ambient room storage, gave good results, with staining over 80% of control levels up to four days after collection. Anthers were easy to manipulate, but became rather brittle by Day 10.
Condition B, desiccator storage, also gave good results, with staining over 80% of control levels up to four days. However, by Day 4, the anthers had dried to a state that made them very difficult to handle. On Day 10, the anthers would shatter when picked up with forceps. Storage in a desiccator with CaSO
4
past four days cannot be recommended.
Condition C, high-humidity storage, was much less successful. The proportion of pollen staining was consistently lower than Conditions A or B, and the anthers deteriorated visibly while in storage. By Day 7, the texture of the anthers became quite flaccid and on Day 10, the anthers were all discolored and soft.
Rhododendron
pollen should not be stored under high-humidity conditions. This finding is in agreement with Visser's (1955) results using
R. catawbiense
Michx. and
R. molle
(Blume) G. Don. High humidity is also detrimental to the survival of the pollen of pearl millet (Sarr etal., 1983), grapes (Randhawa etal., 1982), and many other species (Visser, 1955). There are some exceptions to this generalization (see Stanley and Linskens, 1974).
|
Analogous to Figure 1, Figure 2 shows the results of the cold-storage tests, Conditions D, E, and F. Condition D, refrigerated storage, gave acceptable results, with treatments varying between 69% and 91% of the control means. No deterioration in anther quality was observed. These results are in contrast to the advice of Bowers (1932), who stated that refrigerated storage was poorly suited for
R. catawbiense
pollen.
Condition E, freezer storage with no desiccation, gave unacceptable results. In four of 36 anthers sampled, no viable pollen was found. Extremely low pollen viability was found in all samples of the 'Alaska' cultivar. 'Alaska' has anthers that appear more moist than those of 'Variegated Dogwood' shortly before and at anthesis. It is probable that high moisture content at freezing causes irreversible damage to the pollen (Stanley and Linskens, 1974; Visser, 1955). Direct freezing cannot be recommended.
Condition F, freezer storage following desiccation, generally gave the best results of all storage conditions (five of six test dates). Schroeder and Bump (1982) and Visser (1955) agree that desiccated, frozen
Rhododendron
pollen will remain viable for at least two years. This study confirms their observations on the necessity of desiccation and recommends this storage method for short-term storage as well. At room temperature, 18 hours under desiccating conditions were sufficient to dry the pollen, as compared with the three to seven days of refrigerated desiccation suggested by Schroeder and Bump(1982).
Conclusion
Six storage methods for holding evergreen azalea pollen up to two weeks were compared. The best method of those tested is storage of samples in a freezer following 18 hours in a desiccator at room temperature. Ambient room conditions could be used for storage of up to four days, but humidity needs to be kept low. Desiccator storage and refrigerated storage gave intermediate results. Whereas high-humidity storage and freezer storage without prior desiccation proved unacceptable.
Acknowledgments
I sincerely thank Doctors Carlos Fear, Richard Hall, and William Roath for their helpful comments and advice in the preparation of this manuscript. Laboratory assistance of Ms. Bobbie Jo Morrell is greatly appreciated.
Literature Cited
Bowers, C.C. 1932. Preservation, storage and artificial germination of rhododendron pollen. Proc. 6th Intl. Congr. Genet. 2: 10.
Hecker, R.J. 1963. Use of tetrazolium salts in determining viability of sugar beet pollen. J. Amer. Soc. Sugar Beet Technol. 12: 521-528.
Lee, F.P. 1958. The Azalea Book. Princeton, NJ: D. Van Nostrand Co.
Randhawa, C.S., P.K. Agarwal, and R. Singh. 1982. Pollen storage studies in grapes. I. Effect of different humidity regimes on viability. Indian J. Hortic. 39: 24-28.
Sarr, A., B. Fraliegh, and M. Sandmeier. 1983. Aspects of reproductive biology in pearl millet,
Pennisetum typhoides
(Burm.) Staph and Hubb.
In
Pollen: Biology and Implications for Plant Breeding, D.L. Mulcahy and E. Ottaviano, eds. New York: Elsevier, pp. 381-388.
Schroeder, H.R. and F.E. Bump. 1982. Pollinating and collecting pollen. J. Amer. Rhod. Soc. 36: 8-9.
Stanley, R.G. and H.F. Linskens 1974. Pollen: Biology, Biochemistry, and Management. New York: Springer-Verlag, 307 pp.
Visser, T. 1955. Germination and storage of pollen. Meded. Landbouwhogesch. Wageningen 55: 1-68.
Widrlechner, M.P. and H.M. Pellett. 1983. Notes on the pollen of deciduous azalea cultivars. J. Amer. Rhod. Soc. 37: 210-212.
____, _____, _____, _____, P.D. Ascher and S.C. Fuhrman. 1983.
In vivo
pollen germination and vital staining in deciduous azaleas. HortSci. 18: 86-88.
Journal Paper No. J-12212 of the Iowa Agriculture and Home Economics Experiment Station, Ames, Iowa. Project No. 1018.
Mark Widrlechner, horticulturist with the USDA-Agricultural Research Service, Ames, Iowa, currently serves on the ARS Research Committee and as a technical advisor to the ARS Journal.