Micropropagation Of Rhododendron chapmanii
Frank A. Blazich, Cheryl C. Giles, and Carole M. Haemmerle
Department of Horticultural Science
North Carolina State University
Raleigh, North Carolina
Reprinted from Journal of Environmental Horticulture, March 1986
Abstract
Shoot tips excised from Chapman's rhododendron (
Rhododendron chapmanii
A. Gray) were surface sterilized, the terminal portions were removed (decapitated) and the shoots placed in liquid Woody Plant Medium (WPM) supplemented with 8M (1.6 ppm) 6-(γ, γ-dimethylallylamino)-purine (2iP). Within 2 to 3 months axillary shoots were excised, decapitated and cultured in agar-solidified WPM supplemented with 49 M (10 ppm) 2iP. Multiple shoot formation consisting of adventitious and axillary shoots was observed within 4 to 6 months. These shoots were transferred to WPM supplemented with 8 M (1.6 ppm) 2iP and cultured under reduced light levels to stimulate shoot elongation. Shoots
>
10 mm (0.4 in.) were harvested (micro-cuttings) and rooted using non-
in vitro
procedures. Enhancement of axillary shoot multiplication was achieved by culturing decapitated axillary shoots under reduced light levels in a horizontal position on WPM supplemented with 8 M (1.6 ppm) 2iP.
Introduction
Chapman's rhododendron (
Rhododendron chapmanii
A. Gray), a rare and endangered species (7, 12) can be propagated by stem cuttings (6). Although this method permits cloning of desirable genotypes, it would be a slow procedure for rapidly increasing a particular clone. However, rapid multiplication of desirable selections might possibly be achieved through tissue culture procedures which have been successfully used on many
Rhododendron
species (1, 2, 5, 8, 10, 11). Since propagation using tissue culture offers distinct advantages in comparison to stem cutting propagation and because
in vitro
techniques appear to be feasible, the following experiments were conducted to develop procedures for micropropagation of Chapman's rhododendron.
Materials and Methods
Experiment 1: Development of a repeatable procedure
. Terminal shoot tips, 2 to 4 cm (0.8 to 1.6 in) in length, were taken from actively growing, containerized stock plants maintained under greenhouse or field conditions. Stock plants were in the adult growth phase and originated from studies conducted to develop procedures for rooting stem cuttings (6). Following shoot tip removal, large leaves were removed and the small expanding leaves left intact. Shoot tips were placed under running tap water for 15 minutes, washed in soapy water for 1 minute and rinsed under tap water for 10 minutes. The explants were then submersed for 15 minutes with gentle agitation in a 10% Clorox solution, (1.05% sodium hypochlorite) containing 0.05% Tween-20, rinsed 3 times in sterile distilled water and the terminal portions and basal 2 to 3 mm (0.08 to 0.12 in) of each shoot was removed.
Shoot tips were placed (1/tube) in 2.5 x 15 cm (1.0 x 5.9 in) test tubes to which were added to an approximate depth of 1 cm (0.4 in) liquid Woody Plant Medium [WPM (9)] supplemented with 8 M (1.6 ppm) 2iP. The pH of the medium was adjusted to 5.2 prior to autoclaving.
Cultures were maintained at 23 2C (73.4 3.6F) under a continuous photoperiod supplied by 40 W cool-white fluorescent lamps. The lamps provided a photosynthetic photon flux density (photosynthetic radiation between 400 and 700 nm) of 83.0 mol/m
2
/s (6.2 klx) plus a radiant flux density (photomorphogenic radiation between 700 and 850 nm) of 16.5 W/m
2
as measured at the tops of the test tubes with a LI COR LI-185A quantum/radiometer/photometer. All light measurements reported herein were recorded with this instrument.
Shoot tips were transferred weekly for the first 2 weeks to fresh medium and then every 2 to 3 weeks until axillary shoots of at least 1.0 cm (0.4 in) were produced which normally took 2 to 3 months.
Axillary shoots were excised from the original explants, terminal portions removed (decapitated) and the shoots placed horizontally into 80 x 100 mm (3.1 x 3.9 in) Pyrex storage dishes containing 100 ml of WPM solidified with 0.6% agar and supplemented with 49 M (10ppm) 2iP (pH 5.2). Tops of the dishes were sealed with Parafilm and cultures were maintained in a culture room at 25 1C (77.0 1.8F) under a 16-hr photo-period supplied by 40 W cool-white fluorescent lamps. The lamps provided a photosynthetic photon flux density (400 to 700 nm) of 56 to 80 mol/m
2
/s (4.3 to 6.0 klx) plus a radiant flux density (700 to 850 nm) of 11.0 to 15.8 W/m
2
as measured at the shelf surface on which the dishes were placed.

|
|
Fig. 1. Pyrex storage jar used to culture tissue and a clump of
micro-cuttings removed from the jar and of sufficient length [ > 10 mm (0.4 in)] for rooting (Experiment 1). |
Cultures were transferred to fresh medium every 4 to 5 weeks and within 4 to 6 months multiple shoot formation was observed consisting of a combination of adventitious and axillary shoots. Clumps of these shoots were transferred to fresh WPM (pH 5.2) solidified with 0.6% agar and supplemented with 8 M (1.6 ppm) 2iP again utilizing as culture vessels the Pyrex storage jars. Cultures were placed in a Percival MB-54 growth chamber maintained at 25 1C (77 1.8F) with a 16-hr photoperiod supplied by 40 W cool-white fluorescent lamps. The lamps provided a photosynthetic photon flux density (400 to 700 nm) of 31 to 43 mol/m
2
/s (2.3 to 3.3 klx) plus a radiant flux density (700 to 850 nm) of 6.3 to 8.9 W/m
2
as measured at the shelf surface on which the jars were positioned. After 4 to 5 weeks the clumps of shoots were transferred to fresh nutrient medium and grown for an additional 8 to 10 weeks to ensure satisfactory height for rooting.
Shoots (micro-cuttings) were harvested and rooted under high humidity conditions in plastic flats (21.6 x 16.5 x 5.1 cm (8.5 x 6.5 x 2.0 in)) containing a sterile rooting medium of 1 peat: 1 vermiculite (by vol.). High humidity was provided by daily syringing of the micro-cuttings with water and enclosure of the flats in sealed plastic bags. Two sets of environmental conditions with respect to light and temperature were utilized for rooting which were virtually identical to those in the culture room and the growth chamber. Percent rooting was noted after 10 to 12 weeks followed by acclimation of the rooted micro-cuttings to reduced humidity and finally acclimation to greenhouse conditions and observance of subsequent growth.
Experiment 2: Enhancement of axillary shoot multiplication. Ten-node axillary shoots stimulated to elongate by transfer to the growth chamber maintained under reduced light levels in comparison to the culture room, were decapitated and were placed horizontally in Pyrex storage jars containing 100 ml of WPM (pH 5.2) solidified with 0.6% agar and supplemented with the following M concentrations of 2iP: 0, 2, 4, 8, 16, and 32 (0, 0.4, 0.8, 1.6, 3.3, and 6.5 ppm 2iP, resp.). Each concentration was replicated 5 times with a replication consisting of a storage jar containing 5 decapitated axillary shoots. Cultures were maintained in the Percival growth chamber under the environmental conditions as described in Expt. 1. After 4 weeks shoots were transferred to fresh medium and left for an additional 10 weeks prior to data collection which included the number and length of all shoots (micro-cuttings) > 5 mm (0.2 in).
Results and Discussion
Similar to what has been reported for other
Rhododendron
species (1, 2, 5, 8, 10, 11), Chapman's rhododendron can be propagated by tissue culture (Fig. 1). Procedures described in Experiment 1 proved to be a reliable means for
in vitro
propagation. Rooting and subsequent acclimation to greenhouse conditions of tissue culture-produced micro-cuttings ranged from 70 to 90%. In actuality, the procedures described in Experiment 1 did not arise from a single experiment but incorporate results of many experiments conducted over a 3-year period.
Two critical steps in propagation of Chapman's rhododendron by tissue culture as outlined in the first experiment involve multiple shoot formation from axillary shoots produced from the original explants (shoot tips) and elongation of the multiple shoots by transfer of these shoots to WPM containing reduced 2iP levels (49 to 8 M (10 to 1.6 ppm)) and subsequent culture under reduced light levels. Commencement of multiple shoot formation, consisting of both adventitious and axillary shoots, generally began 4 to 6 months after axillary shoots were excised from the original explants and transferred from the test tubes to the storage jars containing agar-solidified WPM supplemented with 49 M (10 ppm) 2iP. Once multiple shoots began to develop their numbers could be increased simply by sub-culturing shoot masses on the same medium and subsequent growth in the culture room. Although light levels in the culture room promoted shoot multiplication, shoots would not elongate to a sufficient length
>
10 mm (0.4 in) to permit easy handling for non-
in vitro
rooting. This was also the case if the 2iP concentration was reduced and sub-cultured shoots maintained in the culture room; the shoots would still not elongate.
Elongation of tissue culture-produced shoots was achieved once the shoots were sub-cultured and maintained in the growth chamber at reduced light levels. Elongation could be achieved whether or not the 2iP concentration was reduced. However, reduction of 2iP from 49 to 8 M (10 to 1.6 ppm) produced micro-cuttings with larger leaves and thicker stems.
Genetic instability has been observed for
Rhododendron
species propagated by tissue culture in which the procedure resulted in the production of adventitious shoots (10). In our work thus far, no aberrant plants have been observed to arise when the procedures outlined in Experiment 1 are followed even though it involves production of both adventitious and axillary shoots. In attempts to avoid this potential problem the second experiment was conducted to maximize axillary shoot production.
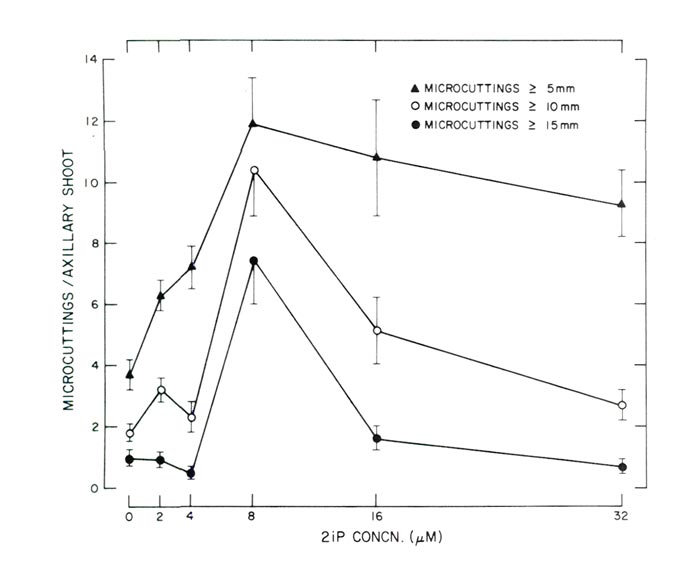
|
|
Fig. 2. Response of decapitated, 10-node axillary shoots cultured in a horizontal
position on agar-solidified WPM supplemented with varying concentrations of 2iP (Experiment 2). Each data point is based on 25 observations and the vertical lines represent 1 standard error. |
In Experiment 2 the greatest number of utilizable micro-cuttings of axillary origin was produced at a concentration of 8 M (1.6 ppm) 2iP (Figs. 2 and 3). Since micro-cuttings were rooted using non-
in vitro
conditions all shoots
>
10 mm (0.4 in) were considered utilizable for this method of rooting. Not only were the greatest number of micro-cuttings
>
10 mm (0.4 in) produced at 8 M 2iP, but the greatest number of micro-cuttings
>
5 mm (0.2 in) and
>
15 mm (0.6) were also produced at the same 2iP concentration (Fig. 2) with reductions at concentrations < or > 8M (1.6 ppm).
Shoot fresh weight data were not recorded in the second experiment since micro-cuttings were used for rooting studies. However, it appeared the heaviest shoots were also produced at 8M (1.6 ppm) 2iP since these shoots had the largest leaves and also the thickest stems.
Some axillary shoots developed on WPM without any 2iP (Fig. 2). The numbers though were few and the shoots were chlorotic and spindly in appearance. Also, at the time data were recorded, cultures maintained on WPM without any 2iP appeared in a state of senescence. Apparently 2iP is necessary for production and uniform growth of axillary shoots.
Response of the micro-cuttings to varying concentrations of 2iP to enhance axillary shoot multiplication is similar to data of McCown and Lloyd (10) for tissue culture propagation of an unidentified cultivar of
Rhododendron
x 'P.J.M.'. Similarities are possibly due to the close taxonomic relationships since
Rhododendron chapmanii
and
Rhododendron
x 'P.J.M.' are both lepidote rhododendrons. Closeness of the botanical relationships is further illustrated by the fact that
Rhododendron chapmanii
is similar taxonomically to
Rhododendron carolinianum
Render (Carolina rhododendron (4)) which is one of the parents of the
Rhododendron
x 'P.J.M.' hybrids (3). Thus, the work reported herein and that of McCown and Lloyd (10) may provide a general scheme for tissue culture propagation of the lepidote rhododendrons.

|
|
Fig. 3. Enhancement of axillary shoot multiplication by
culturing a decapitated axillary shoot in a horizontal position on agar solidified WPM supplemented with 8μM (1.6 ppm) 2iP (Experiment 2). This particular cluster of axillary shoots consisted of 10 micro-cuttings between 15 and 30 mm (0.6 to 1.2 in) in length and an additional 6 micro-cuttings ranging from 5 to 12 mm (0.2 to 0.5 in). |
Micro-cuttings of Chapman's rhododendron were relatively easy to root. Provided desiccation was avoided, percent rooting often approached 100% with the percentage of greenhouse-acclimated plants always being less. Ease of rooting may possibly be related to a reversion to juvenility since tissue culture-propagated plants had morphological features similar to seedlings despite the stock plants being in the adult growth phase.
Successful rooting of micro-cuttings was achieved under light and temperature conditions identical to those in the culture room and the growth chamber. Although studies were not conducted to determine optimum rooting conditions, it appeared that faster rooting and development of more uniform and heavier root systems occurred if cuttings were rooted under light levels similar to those in the culture room. In addition, a preliminary study has indicated that light levels higher than those in the culture room may be still better for rooting.
Once micro-cuttings were rooted and acclimated to greenhouse conditions it was necessary to cut back the plants to promote lateral branching. Chapman's rhododendron has a semi-indeterminate growth habit and similar growth was observed with tissue culture propagated plants. If plants were not pinched they tended to grow as a single stem.
Results reported demonstrate that Chapman's rhododendron can be propagated by tissue culture. This should aid recovery efforts and promote introduction of particular selections. However, individuals are cautioned about collection of plant material from native populations since the plant is a rare and endangered species (7, 12) which severely restricts exploitation to promote conservation. Cultivated seedling material is currently available from several sources. Tissue culture propagation of these plants should be feasible using the procedures described.
Significance to the Nursery Industry
Two procedures, one involving production of adventitious and axillary shoots and the other just axillary shoots, are outlined which provide a means to propagate Chapman's rhododendron by tissue culture. Although both procedures will produce large numbers of easily rooted micro-cuttings, it is extremely important that the shoots produced
in vitro
, which will be eventually harvested as micro-cuttings, be cultured under light levels (31 to 43 mol/m
2
/s (2.3 to 3.3 klx) to stimulate satisfactory shoot elongation. Shoot elongation is critical because one needs to produce micro-cuttings which are of sufficient length
>
10 mm (0.4 in) to permit easy handling for non-
in vitro
rooting. In addition, the procedures described, with or without modification, may be useful for propagating other lepidote rhododendrons by micropropagation.
For information on "Propagation of Rhododendron chapmanii by Stem Cuttings", see William Gensel and Frank Blazich's article,
ARS Journal
, Vol. 40:2 (Spring 1986).
Literature Cited
1. Anderson, W.C. 1975. Propagation of rhododendrons by tissue culture: Part 1. Development of a culture medium for multiplication of shoots. Proc. Intern. Plant Prop. Soc. 25:129-135.
2. Anderson, W.C. 1978. Rooting of tissue cultured rhododendrons. Proc. Intern. Plant Prop. Soc. 28:135-139.
3. Dirr, M.A. 1983. Manual of Woody Landscape Plants: Their Identification, Ornamental Characteristics, Culture, Propagation and Uses. 3rd ed. Stipes Publishing Co., Champaign, IL.
4. Duncan, W.H. and T.M. Pullen. 1962. Lepidote rhododendrons of the southeastern United States, Brittonia 14:290-298.
5. Fordham, I., D.P. Stimart and R.H. Zimmerman. Axillary and adventitious shoot proliferation of Exbury azaleas
in vitro
. HortScience 17:738-739.
6. Gensel, W.H. and F.A. Blazich, 1985. Propagation of
Rhododendron chapmanii
by stem cuttings. J. Environ. Hort. 3:65-68.
7. Greenwalt, L.A. 1979. Determination that
Rhododendron chapmanii
is an endangered species. Federal Register 44:24248-24250.
8. Kyte, L. and B. Briggs. 1979. A simplified entry into tissue culture production of rhododendrons. Proc. Intern. Plant Prop. Soc. 29:90-95.
9. Lloyd, C. and B. McCown. 1980. Commercially-feasible micropropagation of mountain laurel,
Kalmia latifolia
, by use of shoot-tip culture. Proc. Intern. Plant Prop. Soc. 30:421-437.
10. McCown, B.H. and C.B. Lloyd, 1983. A survey of the response of
Rhododendron
to
in vitro
culture. Plant Cell, Tissue and Organ Culture 2:77-85.
11. Meyer, M.M. Jr. 1982.
In vitro
propagation of
Rhododendron catawbiense
from flower buds. HortScience 17:891-892.
12. Simons, R.W. 1983. Recovery plan for Chapman's rhododendron
, Rhododendron chapmanii
A. Cray. U.S. Fish and Wildlife Service. Atlanta, GA. 41p.