Seasonal Changes In Anther Development Of Cold Hardy Rhododendrons
Wilmer C. Stowe, Charles V.M. Fink and David G. Leach
North Madison, Ohio
Introduction
Plant biotechnology is giving plant breeders new tools to increase hybridization precision and produce homozygous plants within one generation. These new methods have the potential for the identification of masked genes and the significant reduction by decades of the time required for the production of woody homozygous breeding parents. One such technique is anther/pollen culture which initially can produce a haploid tissue mass and subsequently regeneration of homozygous plants by doubling the haploid chromosome number. The authors' initial goal was to develop by tissue culture haploid rhododendron tissue and subsequently haplodiploid plants. These doubled haploid (haplodiploid) rhododendrons will be mature homozygous plants produced in a few years rather than decades of intensive breeding.
Nitsch (1981), Dodd and Roberts (1982) and others stated that there are several times during pollen development when the tissue is very plastic and suitable for in-vitro cultivation. Thus our first goal was the identification of the stages in rhododendron pollen development. This is a report of our efforts to track pollen development and identify the stages most suitable for in-vitro development.
One notable difference between our research and earlier work is that we were working with woody plants, which take several months to complete pollen development. Earlier work has concentrated on annual plants, which may complete pollen development in a few weeks. Because the data presented here are the results of one year's observations, the possibility of some seasonal variability must be considered for other years. Unlike Widrlechner et al. (1983) who tied azalea development to photoperiod and accumulated temperature effects, our only variable was time of year.
To date major research on
Rhododendron
flowers has concentrated on: pollination (Williams et al., 1986), pollen ecology (Rouse et al., 1986) and development (Kaul et al., 1987 and Palser, 1985). In this study we tracked microsporogenesis in three cold hardy elepidote rhododendron cultivars from formation of diploid sporogenous tissue, to the haploid microspores and finally to a binucleate microspore (pollen grain). These results are being used in our anther culture efforts. These results should also give other breeders floret and anther markers useful in identifying optimum stages of anther development for isolation and culture of haploid plant tissue. As of January 1, 1989, haploid tissue in viable culture have not been achieved; however cell nuclei with irregular chromosomal numbers were observed in some callus tissue. This is a promising indication that the goal can be achieved.
This research was supported in part by a grant from the American Rhododendron Society Research Foundation and by generous funding by Mr. Edward Losely, nurseryman of Perry, Ohio. Mr. Losely also contributed laboratory space, equipment and professional technicians in his tissue culture laboratory to support this project. We should also like to thank Charles Tubesing and Peter Bristol of The Holden Arboretum for their review of this manuscript.

|
Materials and Methods
The three Leach hybrid rhododendrons used in this study were R. 'Burma' (R.H.S. Dec. 19, 1983), R. 'Monaco' (ARS Journal, 1984, Spring) and R. 'Nepal' (not registered). Descriptions of these three cultivars can also be found in Salley and Greer (1986). These plants were grown outdoors at the David C. Leach Rhododendron Research Station of The Holden Arboretum in an uncontrolled environment. During the study the subject plants received no added fertilizers or watering.
Flower buds from those three rhododendron hybrids were collected from July 27, 1986 through bloom time in the spring, 1987. Four to six buds were randomly collected from each hybrid on a weekly or monthly basis depending on the season and physiological state of the plant. Weekly samples were taken during the late summer, early fall and spring and monthly samples were taken during winter dormancy for the cultivars R. 'Monaco' and R. 'Nepal'. Samples of R. 'Burma' were regularly taken during the early and later part of this study and irregularly during the late fall and winter months.
Buds were measured then fixed for 24 hours in modified Carnoys solution after which they were stored in 70% ethyl alcohol at 4 C (Evans & Reed, 1981). Florets and anthers were dissected later for study. Depending on their size the florets and anthers were measured with the aid of a calibrated stereoscopic or compound microscope. All measurements were in millimeters or converted into millimeters. The number of florets and anthers measured for each cultivar is as follows: R. 'Burma' (100, 264), R. 'Monaco' (184, 825) and R. 'Nepal' (291, 604), respectively.
Cytological state of development for anther tissue or pollen grains was determined by the acetocarmine squash technique (Evans & Reed, 1981). We examined the largest and smallest floret in a single bud to determine developmental stage; if both were in the same stage of development we assumed all other florets to be in the same developmental stage and went on to the next bud. However, if the anthers from the largest and smallest floret from a single bud were not in the same stage of development several more if not all the florets from that bud were examined. Cytological stages of development were similar to the stages observed by Raghavan (1988).
Results & Discussion
Through acetocarmine squashes nine cytological stages of anthers/ pollen development were observed from bud initiation to flowering. The major characteristics of these nine stages are described in Table 1. The most obvious cytological changes are those from stage II through stage V. Unlike most other flowering plants the microspores of the Ericaceae remain attached to each other as a tetrad of microspores. This tetrad remains intact even after flowering. Stage V is usually complete after 30 to 45 days of obvious bud initiation.
Starch grain formation (stage VI) as the four segments of the pollen tetrad develop starts very inconspicuously as microstarch grains less than one micrometer (1/1,000 of a mm) in diameter. Starch grain development will occur over a three to five month period. Approximately four weeks prior to flowering, these starch grains will have increased to nearly five micrometers in diameter. Generally the division of the single nucleus (stage VII) in the microspores to form true pollen grains, occurred three to four weeks prior to flowering. However, this nuclear division was not observed in all microspores even up to the time of pollen release from the anther. It is possible that microspore nuclear division may not occur in all of the microspores of a single tetrad. It is also possible that the large number of starch grains may be obscuring the second nuclei in some microspores of the pollen tetrads (Palser, personal communication).
| Table 1. Stages of cytological development in anthers of Rhododendron cultivars 'Burma', 'Monaco', and 'Nepal'. | |
| Stages Cytological condition of the developing anther. | |
| I | Anther contains earliest pollen precursor cells (sporogenous tissue). Anther wall cells are essential nurse tissue. Anther and contents are diploid. |
| II | Individual cells of sporogenous tissue develop into microspore mother cells. Translucent sheath developing around individual microspore mother cells. |
| III | Diploid stage preceding reduction division to haploid state, identified by a translucent sheath around individual microspore mother cells. (Leach 1988 figs. 3 & 4.) Reductional division in progress. |
| IV | Reductional division producing haploid cells within the translucent sheath. This division produces four haploid cells in a tetrad. (Leach 1988 fig. 5.) |
| V | Completing tetrad formation; pollen wall develops; translucent sheath being absorbed. |
| VI | Starch granules become apparent in haploid microspore cytoplasm. |
| VII | Microstarch granules enlarging. Nuclei of some microspores divide by mitosis to complete pollen formation. |
| VIII | Flower buds begin to break. |
| IX | Flowering complete. |
| Our laboratory experiments indicate that stage VII may be the most plastic stage in anther/pollen development. Therefore this may be the most appropriate stage for in-vitro culture of haploid tissue. | |
Figure 1 shows seasonal changes in average anther size and developmental stage for the three cultivars from July 27, 1986 through flowering in spring, 1987. This figure shows a rapid increase in anther size for about the first forty-five days following apparent bud initiation and about twenty to thirty days prior to flowering with a period of little size change during the winter dormancy period. While growth during late summer, early fall and spring periods with dormancy during the winter is expected, the relative closeness in anther size for all three cultivars was not anticipated.
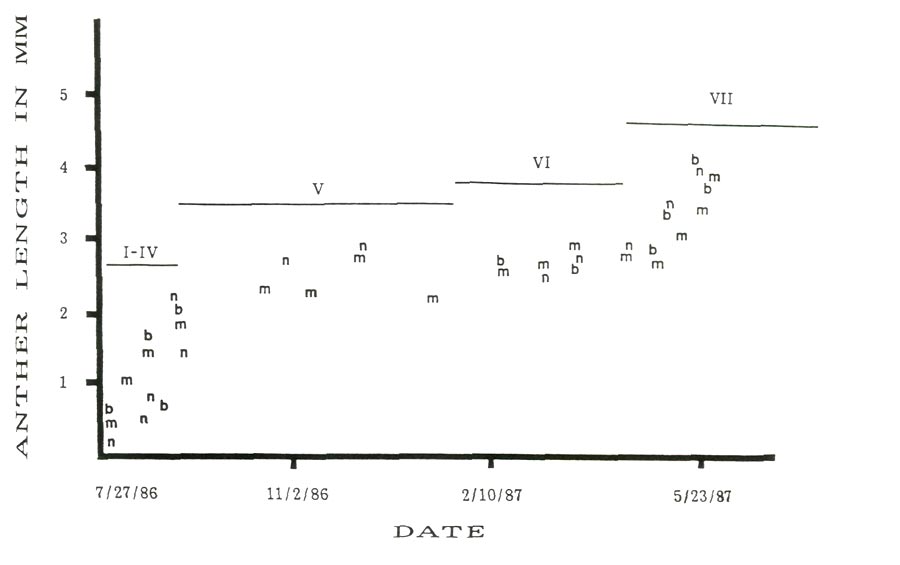
|
|
Figure 1: Seasonal changes in anther length with cytolical stages,
'Burma' = b, 'Monaco' = m and 'Nepal' = n. |
Rhododendron 'Monaco' and R. 'Nepal' might be expected to be similar in development because
R. catawbiense
var.
album
is the seed parent of both cultivars. The species components of the pollen parents for both cultivars are closely related phylogenetically. There are no surviving records on the ancestry of the parents of R. 'Burma'.
Figure 2 is a scatter plot of average anther size versus average floret size with the approximate stage of anther development for the three cultivars studied. In all three cases there is a linear relationship between anther and floret size for florets less than 4.5 mm and greater than 5.5 mm. The anthers in the corresponding florets are less than 1.5 mm or greater than 2.2 mm. The observed jump occurs in the anther size range (1.5-2.2 mm) in which reduction division and pollen wall formation are taking place (stages IV and V). Between these two floret sizes there is a rapid increase in anther size relative to the floret size. The linear increases for all cultivars before and after the size jump are almost parallel. Thus microspore enlargement, reductional division and the formation of the pollen wall would appear to be positively correlated to the rapid expansion of the anthers at this time.
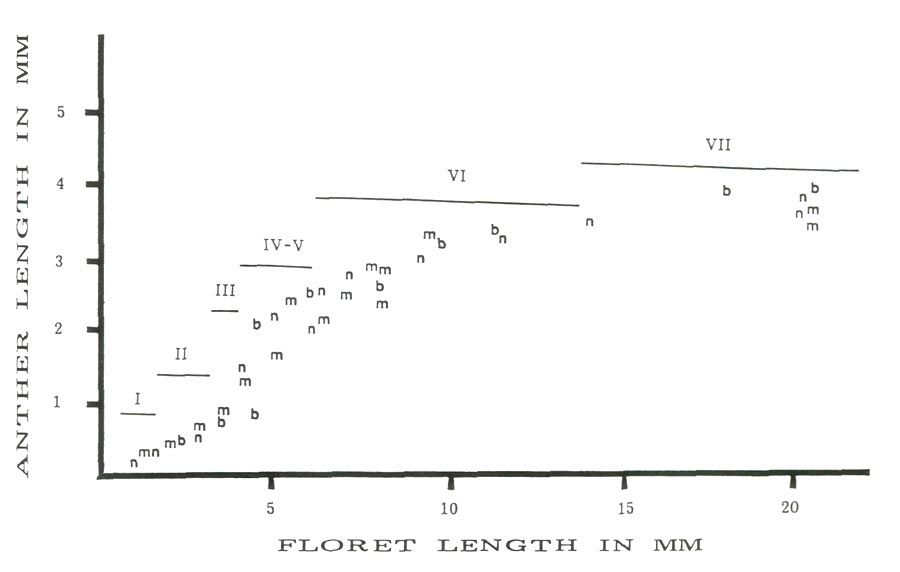
|
|
Figure 2: Anther length vs. floret length with cytolical stages,
'Burma' = b, 'Monaco' = m and 'Nepal' = n. |
Table 2 presents the anther and floret length range along with cytological developmental stage for eight major time intervals. The most important point to be made here is the extreme variation in anther and floret size. This variation in size range is most dramatic during the earlier stages of development. These results are consistent with those of Widrlechner et.al. (1983) who observed a great variation in developmental timing among and within azalea cultivars.
In late August and early September the initial size relationship of anther to cytological developmental stage showed that anthers approximately 1.6-1.8 mm long were in stage IV for all three cultivars. Anthers that were 2.1 mm long or longer were in stage V. Also in late August to early September anthers collected from florets in a single bud may show a size range of 0.60 to 2.5 mm with the largest anthers being found in the florets at the base of the rachis and the smallest anthers being found in florets at the tip of the rachis. However by mid to late September all anthers of all cultivars were in stage V regardless of anther size. In late September several anthers 1.5 mm long were observed to be in late stage V. There is no synchrony in cytological development for many of the earlier buds of all the cultivars. Prior to late September the florets within a single bud may show anthers in developmental stages I through V. After late September all anthers possessed well formed tetrads each with heavy pollen walls.
The winter dormancy is really a misnomer since it is at that season that starch accumulation begins by the formation of microstarch granules (stage VI). The enlargement of the microstarch granules takes place during the traditional winter season and is completed by flowering.
| Table 2. Cytological stages of development relative to anther and floret length for the cultivars 'Burma', 'Monaco' and 'Nepal'. | ||||
| Hybrid | Time Span | Developmental Stage |
Anther Length Range
in mm |
Floret Length Range
in mm |
| 'Burma' | July 27-Aug. 11 | I | 0.53 - 1.20 | 2.4 - 4.0 |
| Aug. 11-Aug. 20 | II | 0.6 - 2.2 | 2.0 - 5.3 | |
| Aug. 20-Sept. 5 | II, III, IV | 0.6 - 2.4 | 3.0 - 4.5 | |
| Sept. 5-Feb. 25 | V? | |||
| Feb. 25-March 1 | V, VI | 2.2 - 2.9 | 5.0 - 7.0 | |
| March 1-April 12 | VI | 2.0 - 2.9 | 4.5 - 7 | |
| April 12-May 10 | VI, VII | 2.5 - 3.8 | 7.0 - 12.0 | |
|
May 10-May 29
Anthesis May 17 |
VIII, IX | 3.0 - 4.5 | 11.0 - open | |
| 'Monaco' | July 27-Aug. 11 | I | 0.26 - 1.25 | 0.75 - 4.2 |
| Aug. 11-Aug. 20 | II, III, IV | 0.40 - 2.3 | 3.0 - 5.0 | |
| Aug. 20-Sept. 5 | II, III, IV | 0.21 - 2.6 | 3.5 - 6.7 | |
| Sept. 5-Oct. 17 | II, III, IV, V | 0.9 - 2.9 | 4.0 - 6.2 | |
| Oct. 27-March 1 | V, VI | 1.3 - 3.1 | 5.0 - 8.0 | |
| March 1-April 12 | VI | 2.3 - 3.4 | 6.5 - 8.0 | |
| April 12-May 10 | VII | 2.4 - 3.9 | 7.5 - 10 | |
|
May 10-May 29
Anthesis May 24 |
VIII | 3.4 - 4.5 | 11 - 16 | |
| 'Nepal' | July 27-Aug. 11 | I | 0.18 - 0.56 | 0.4 - 2.0 |
| Aug. 11-Aug. 20 | I, II | 0.18 - 1.2 | 1.1 - 5.0 | |
| Aug. 20-Sept. 5 | I, II, III, IV | 0.58 - 2.5 | 1.1 - 6.0 | |
| Sept. 5-Oct. 17 | III, IV, V | 0.7 - 2.4 | 2.8 - 5.2 | |
| Oct. 27-March 1 | V | 1.9 - 3.2 | 5.0 - 7.0 | |
| March 1-April 12 | VI | 2.3 - 3.1 | 5.0 - 6.0 | |
| April 12-May 10 | VII | 2.5 - 4.0 | 7.0 - 12.0 | |
|
May 10-May 29
Anthesis May 17 |
VIII, IX | 3.0 - 4.0 | 15.0 - open | |
A point of interest to us is why should a plant produce haploid tissue prior to the onset of winter? Is there greater survivability of the haploid pollen tetrad as opposed to normal diploid? The florets remain soft during the winter while other vegetative tissue hardens off prior to winter. This may be the reason flower buds are more sensitive to damage by winter cold than any other part of the rhododendron.
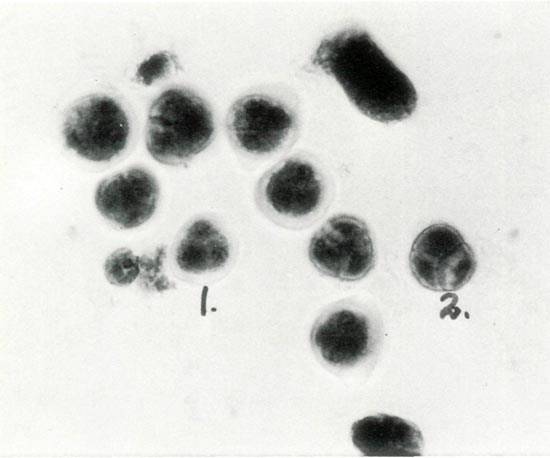
|
|
Figure 3. Photomicrograph of Stage V: the tetrad labeled No. 1
shows a translucent sheath, which has been digested in tetrad No. 2 where the exine wall is thickening. Photos by Stowe/Fink/Leach Haploid Rhododendron Research Project. |
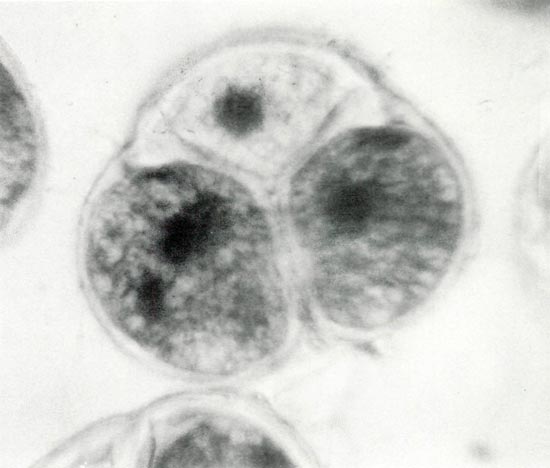
|
|
Figure 4. Photomicrograph of a mature pollen tetrad showing the
darkly stained binucleate state in two of the three cells. The fourth cell is out of the plane of view. The presence of starch grains produces the finely divided reticulate appearance. Photos by Stowe/Fink/Leach Haploid Rhododendron Research Project. |
While this article centers around pollen development, our goal is the production of doubled haploid (haplodiploid) plants. Our laboratory work indicates that the time of nuclear division within the pollen grain (stage VII) is the most plastic stage of development. It is during stage VII that we observed the greatest morphological changes in cultured pollen tetrads. Our pollen developmental studies have given us size and cytological markers enabling the easy identification of this stage.
Bibliography
Dodds, J.H. & L.W. Roberts. 1982. Anther and pollen culture.
Experiments in Plant Tissue Culture
. Cambridge U. Press, pp. 127, 140.
Evans, DA and S.M. Reed. 1981. Cytogenetic Techniques.
Plant Tissue Culture
. Academic Press, Inc. pp. 213-241.
Kaul, V., C.H. Theunis, B.F. Palser, R.B. Knox and E.G. Williams. 1987. Association of the generative cell and vegetative nucleus in pollen tubes of
Rhododendron. Annals of Botany
59:227-235.
Leach, D.G. 1988. The nature of evidence for hybridity in
Rhododendron yakushimanum
'Mist Maiden', 'Pink Parasol' and 'Ken Janeck'.
Journal of the American Rhododendron Society
. 42:(4) 207-209.
Nitsch, C. 1981. Production of isogenic lines: Basic technical aspects of androgenesis.
Plant Tissue Culture, Methods & Applications in Agriculture
. Ed. T.A. Thorpe. Academic Press, pp. 241-252.
Palser, B.F. 1985.
Rhododendron
an intimate glimpse into the flower.
Journal of the Australian Rhododendron Society
. 24:(4) 52-70.
Raghavan, V. 1988. Anther and pollen development in rice (
Oryza sativa
).
Amer. J. Bot.
75:183-196.
Rouse, J.L., E.C. Williams and R.B. Knox. 1986. Floral features related to pollination ecology in
Rhododendron
.
Proceedings of Symposium Pollination 86
. University of Melbourne.
Salley, H.E. and H.E. Greer. 1986.
Rhododendron Hybrids: A Guide to Their Origins
. Timber Press. Portland, Oregon.
Widrlechner, M.P., H.M. Pellett and P.D. Ascher. 1983. The timing of microsporogenesis in deciduous azaleas.
Journal of the American Rhododendron Society
. 37:291-94.
Williams, E.G., V. Kaul, J.L. Rouse and B.F. Palser. 1986. Overgrowth of pollen tubes in embryo sacs of
Rhododendron
following interspecific pollinations.
Aust. J. Bot
. 34:413-423.