'Little Pioneer': A Vireya-Rhododendron Hybrid
John Rouse
University of Melbourne
Parkville, Victoria, Australia
Os Blumhardt
Whangarei, New Zealand
Introduction
The genus
Rhododendron
L. contains some 900 species and is one of the largest angiosperm genera.
Rhododendron
inhabit much of the northern hemisphere where they are distributed from within the arctic circle to the equatorial tropics, the most southerly species being
R. lochiae
which is found on isolated mountain tops in northeastern Australia. The species display a wide range of morphological characteristics. They vary in size from low, spreading mats no more than 10 cm high to trees taller than 20 m, their leaves range in length from 5 mm to 500 mm and their flowers range in size from less than 10 mm long to more than 100 mm. While mostly evergreen, there are about 50 deciduous species, and though many species grow terrestrially, they are frequently found growing epiphytically on tall trees particularly in the tropics.

|
As might be expected with this wide range of characteristics, there are barriers to hybridization in the genus. These breeding barriers frequently occur between the major taxonomic subdivisions (Williams et al., 1985), but may also arise within a taxon due to disparate flower size (Williams and Rouse, 1988). In the wild, hybridization is naturally impossible between two geographically isolated species. In particular, there is a strong breeding barrier between the two major subgroups, subgenus Rhododendron , the lepidotes, and the other 7 subgenera, the elepidotes, which includes subgenus Hymenanthes and the azalea complex so that attempts to produce lepidote-elepidote hybrids usually fail. On the occasions when success has been reported, the resulting hybrids are rather unattractive in leaf and flower, lack vigour and are sterile (Kehr 1977; Rouse et al. 1988a, 1988b). Within the lepidotes there is a surprisingly strong breeding barrier between section Rhododendron and section Vireya (Williams et al., 1985). We know of no reports of crosses spanning this barrier which resulted in seedlings whose hybridity has been authenticated other than the hybrid R. 'Little Pioneer' = R. lochiae x R. virgatum shown in Fig. 1 and reported by Blumhardt (1984), Rouse (1989) and Williams et al. (1990). Here we give descriptions of the hybrid and its parents and report on our repetition of the cross.

|
|
Figure 1: R. 'Little Pioneer'. This cross,
R.
lochiae x R. virgatum was made by O. Blumhardt in 1964. The plant looks more like the male parent and it appears to be completely sterile. Photo by Michael Cullinane |
The Female Parent,
R. lochiae
Mueller
Rhododendron lochiae
was collected near the summit of Mt Bellendenker in northeastern Queensland in 1886 on the first ascent by Sayer and Davidson (Sayer, 1888) and described and named by Ferdinand Von Mueller (Mueller, 1887). This century
R. lochiae
has been collected near the summit of other Queensland mountains including Bartle Frere, Mt Spurgeon, Mt Lewis, Thornton Peak shown in Fig. 2 and Mt Finnigan whose heights range between 1,100 and 1,600 m (3,600 and 5,250 ft). It is the only Australian rhododendron as yet discovered.

|
|
Figure 2: Thornton Peak, 1374 m, in the Cape Tribulation National
Park, northeastern Queensland. The photograph was taken from just south of the Daintree river. As frequently occurs, the summit is enveloped in cloud. The crop in the foreground is sugar cane. Photo by J. L. Rouse |
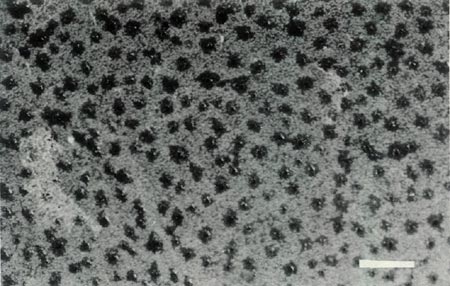
Rhododendron lochiae is an evergreen lepidote shrub placed taxonomically in subgenus Rhododendron , section Vireya , subsection Euvireya , series Javanica by Sleumer (1949, 1966). The leaves are arranged in pseudo whorls and Fig. 3a shows the scales on the underside of a mature leaf. The inflorescence is terminal, usually 2-5 flowered and unscented. The corolla is bright red, tubular and 5-lobed with no visible "bee guide." Under UV-A illumination the face of the corolla is reflecting with a small central absorbing region within the tube and the rear of the corolla shows an unusual and irregular asymmetric patterning (Rouse et al., 1986). The red tubular corolla with UV reflectance and small central UV "bee guide" suggests that R. lochiae is pollinated by birds and/ or insects. The flowers have 10 stamens, the pollen is in tetrads with a diameter of 60 um and the pistil is 5-partite with a length of 30 mm. Figure 4a shows the ovary with a dense covering of scales and hairs, the calyx which is little more than an irregular rim and a gradually tapering style-ovary junction. Figure 5 shows the whole pistil. The seeds have a long tail at each end (Hedegaard, 1980), the seedlings have glabrous cotyledons and the first foliage leaves have juvenile scales mostly around the rim. As with all Vireyas investigated, R. lochiae is diploid, 2n=26 (Janaki Ammal et al., 1950). Colour photographs of R. lochiae can be seen in Headlam (1976), Williams and Rouse (1986) and Smith (1989). A sketch can be seen in Bailey (1900) and a detailed description with a painting is available in Hutchinson (1943).
|
|
|
|
Figure 3: (a) Scales on the underside of
a mature leaf of R. lochiae . Photo by J. L. Rouse |
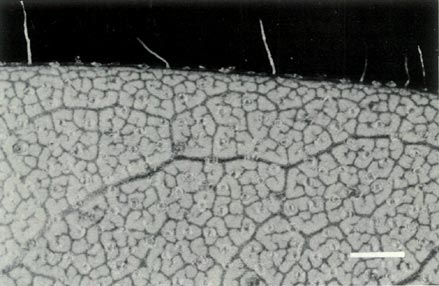
Figure 3: (b) Non overlapping scales on the
underside of a leaf of R. virgatum . Photo by J. L. Rouse |
|
|
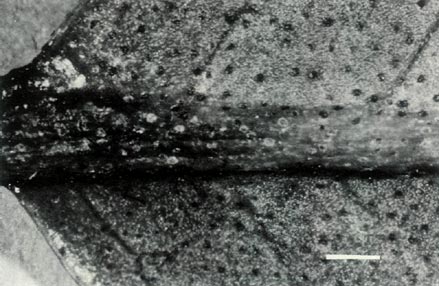
Figure 3: (c) The underside of a mature leaf of
R. 'Little Pioneer'. The entire sessile scales are well spaced on the blade and the raised midrib. The dimensional bars represent a length of 1 mm. Photo by J. L. Rouse |
Rhododendron lochiae is self-compatible and readily hybridizes with other Vireya provided the parental style lengths are not too dissimilar (Williams and Rouse, 1988;Rouseand Williams, 1989) and many horticultural interspecific hybrids have been produced, e.g. R. macgregoriae x R. lochiae and R. lochiae x R. leucogigas 'Hunstein's Secret' (Williams and Rouse, 1990) and R. 'Liberty Bar' = R. aurigeranum x R. lochiae (Williams and Rouse, 1986). Characteristically, F 1 R. lochiae hybrids have red or pink corollas.
The Male Parent,
R. virgatum
Hooker
Rhododendron virgatum
was discovered by J.D. Hooker at 2,500 m (8,200 ft) in Sikkim in 1849 and introduced into cultivation in England the following year. It is a very variable species and widely distributed in Nepal, Sikkim, Bhutan, Assam, Tibet and Yunnan.
Rhododendron virgatum
is an evergreen lepidote shrub taxonomically placed in subgenus
Rhododendron
, section
Rhododendron
, subsection Virgata (Cullen, 1980). This subsection is monotypic and two subspecies are recognized, the Himalayan form subspecies
virgatum
and the Chinese form subspecies
oleifolium
which has slightly smaller flowers. The leaves are scattered up the stem and Fig. 3b shows the scales on the underside of a young leaf and with a few pointed hairs on the rim. The flowers are borne in the axils of the upper leaves with 1 or 2 unscented flowers per leaf axil. The corolla is funnel shaped, 5-lobed and ranges in colour from white through pink to light purple or mauve and with no visible "bee guide". Figures 6a and b shows a flower prior to opening and one fully open. Examination of the corolla under UV-A illumination shows it to be strongly absorbing all over, both inside and outside. The absence of a visible or UV "bee guide" and perfume and the corolla colouring and shape suggests that
R. virgatum
is pollinated by birds. The flowers have 10 stamens, the pollen is in tetrads with a diameter of 55 um and the pistil is 5-partite with a length of 35 mm. Figure 4b shows the scaley ovary with an abrupt transition between it and the style, the 5-lobed calyx with scales on the outside and the top of the pedicel lightly covered with scales. Figure 5 shows the whole pistil. The seeds are un-winged and have a tail at each end (Hedegaard, 1980). They are very similar to some
Vireya
seeds, their tails being as long as those of
R. kawakamii
and longer than those of
R. retusum
though much shorter than the long fine tails on seeds of
R. santapaui
and
R. malayanum
(Rouse, 1985). The seedlings have occasional juvenile scales on the cotyledons and the first foliage leaves have scales around the rim and on the blade (Hedegaard, 1980). Most of the polyploid groups of species are in section
Rhododendron
(Janaki Ammal, 1950) but
R. virgatum
is diploid (Janaki Ammal et at., 1950).
|
|
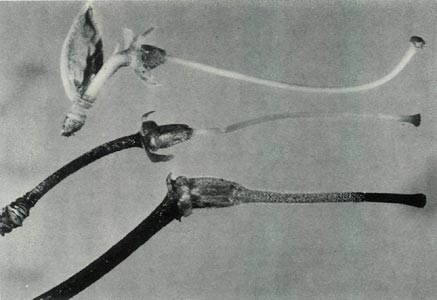
Figure 5: The pistils:
R. virgatum
at the top,
R. 'Little Pioneer' in the centre and R. lochiae at the bottom. Photo by J. L. Rouse |
Subsections
Virgata
and
Scabrifolia
are characterized by axillary inflorescences as distinct from the terminal inflorescences in other section
Rhododendron
subsections. Because of this, Sleumer (1949) classified this group into subgenus
Pseudorhodorastrum
distinct from subgenus
Rhododendron
. However Cullen (1980) considers that in spite of their axillary inflorescence this group of plants should be treated as a subsection of section
Rhododendron
because of their similarity to two other subsections,
Cinnabarina
and
Tephropepla
.
Further descriptions of
R. virgatum
are given in Davidian (1982) and with drawings in Stevenson (1947), Cox (1985) and Young and Chong (1980). Colour photographs of both subspecies
virgatum
and
oleifolium
can be seen in Feng (1988). A colour painting with description can be found in Curtis' Botanical Magazine, Volume 84, Tab 5060 for 1858.
Rhododendron virgatum
is self-compatible but reports of hybrids with any other species are rare. Synge and Platt (1964) list only two F
1
hybrids
R. virgatum
x
R. ciliatum
=
R.
'Multiflorum' and with less certainty
R. virgatum
x
R. hirsutum
=
R.
'Pallidum'. Bulgin (1986) lists no F
1
R. virgatum
hybrids. Whether this lack of recorded hybrids is due to this species not being tried as a parent or because it does not readily cross with other section
Rhododendron
species we do not know.
|
|
|
|
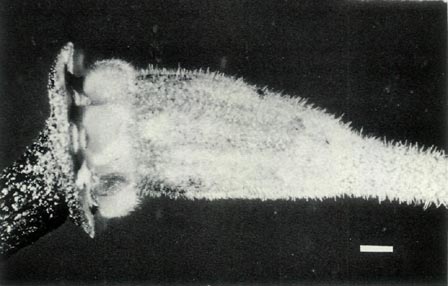
Figure 4: (a) The ovary and base of the style of
R.
lochae showing a covering of scales and fine hairs and a gradual transition at the style-ovary junction. The calyx is little more than a corrugated rim and it and the top of the pedicel are scaley and without hairs. Photo by J. L. Rouse |
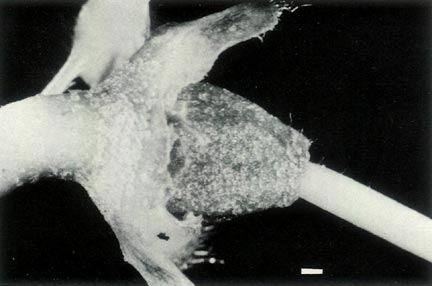
(b) The ovary, style base and 5-lobed calyx of
R.
virgatum . The ovary is densely covered with scales and a few fine hairs and there are scales on the calyx and pedicel. There are a few very fine hairs at the base of the style and the style-ovary junction is abrupt. Photo by J. L. Rouse |
|
|
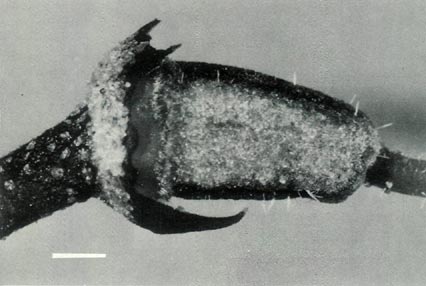
Figure 4: (c) The ovary, style base and calyx of R.
'Little Pioneer'. The ovary is densely covered with scales and a few pointed hairs, and the style base has a few pointed hairs. The style-ovary junction has a slight taper but is still well defined. The calyx and pedicel are scaley. The dimensional bars represent a length of 1 mm. Photo by J. L. Rouse |
The Hybrid;
R.
'Little Pioneer'
In the autumn of 1964 Blumhardt pollinated a plant of
R. lochiae
with pollen of
R. virgatum
obtained from an out-of-season bloom and then a day or two later self-pollinated the same flowers in order to set sufficient selfed seeds to prevent the capsules from abscising before the hybrid seeds, if any, were ripe. This technique is likely to produce a large number of selfed seedlings and rather few hybrid seedlings so it was most fortunate that one of the seedlings was an obvious hybrid. Though it initially lacked vigour it developed into a plant that first flowered about 1967 when only 12-18 cm across and growing in a 10 cm pot. Unfortunately the plant was prone to root rot and required frequent propagating by rooting cuttings. No attempts have been made to propagate it by grafting so we do not know if the hybrid is compatible on either sect.
Vireya
or sect.
Rhododendron
root stock, however, trial graftings are planned.
The hybrid is a lepidote, evergreen, frost tender shrub, Fig. 1, which at first glance looks more like the male parent,
R. virgatum
than the female parent. The leaves are, however, mostly arranged in pseudo-whorls and Fig. 3c shows sessile scales on the underside of a mature leaf whose midrib is raised. The scales are also present on the petiole. The upper surface of this leaf is hairless and when mature lacks scales. Flowering occurs mainly in spring and the flowers are borne in the axils of the upper leaves and terminally. Each cluster has 2-3 bell shaped, orchid pink, scentless, 5-lobed flowers, with no visible "bee guide." One such flower is shown in Fig. 6c. On the outside of the corolla there are fine pointed hairs at the base, inside the corolla is glabrous. The pedicel has a covering of entire sessile scales and short pointed hairs. Under UV-A illumination, the corolla appears absorbing all over both inside and outside. The flowers have 10 stamens and the pistil is 5-partite with a length of 32 mm. Figure 4c shows the ovary densely covered with scales and a few pointed hairs, a scaley pedicel and style base with pointed hairs. The calyx has scales on the outside and the sepals range in size from virtual absence to moderately large both for a particular flower and from flower to flower. The style-ovary junction is slightly tapered. Figure 5 shows a photograph of the hybrid pistil together with a pistil from each of the parents. The in between nature of the hybrid can be seen in the size and shape of the calyx and ovary, the form of the style-ovary junction and even in the colouring of the components.
The hybrid is apparently sterile since using it as a male or female parent has not resulted in seeds. Its male sterility can be inferred from its pollen which is in shriveled tetrads 32-36 um in diameter, which is less than the diameter of any viable
Rhododendron
pollen measured by Williams and Rouse (1990).
Koromiko Nursery had plants of
R.
'Little Pioneer' for sale in 1987, but due to a severe outbreak of rust in the nursery, recent propagations have been destroyed.
|
|
|
|
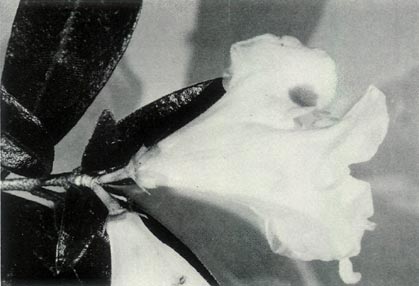
Figure 6: (a)
R. virgatum
just prior to
the flower opening. Photo by J. L. Rouse |
Figure 6: (b)
R. virgatum
, a fully open flower.
Photo by J. L. Rouse |
|
|
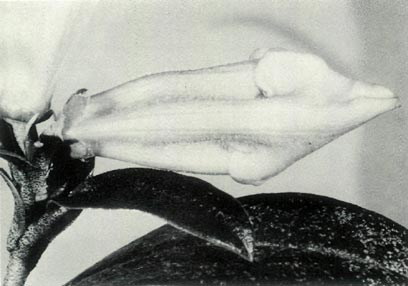
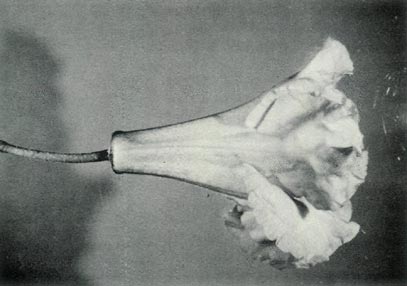
Figure 6: (c) The pedicel and fully open
flower of R. 'Little Pioneer'. Photo by J. L. Rouse |
Repetition of the Cross
In order to discover if the cross could be successfully repeated with just normal pollination techniques, the pollination of
R. lochiae
x
R. virgatum
was repeated by Rouse in the spring of 1988 using a vigorous form of
R. lochiae
from Thornton Peak and pollen of
R. virgatum
collected in the Rhododendron Garden, Olinda, on the November Rhododendron Show weekend with the assistance of Peter Damman, the Director of the Garden. The
R. lochiae
flowers were emasculated with care so that the stigmas were uncontaminated with self-pollen and the
R. virgatum
pollen was stored for some weeks (Rouse, 1984) until the
R. lochiae
stigmas became receptive. The pollination was made only with
R. virgatum
pollen: there was no follow up pollination with
R. lochiae
pollen or treatment of the pedicel base to assist with the retention of the capsules on the plant to seed maturity. At the same time, control pollinations were made of
R. lochiae
selfed and an intraspecific cross with pollen from a plant of
R. lochiae
from a different mountain top was made primarily for seeds. Un-pollinated control pistils were also kept. No apomictic seed set was expected as there are no reports of apomixis occurring in
Rhododendron
. Figure 7a shows a hybrid capsule and Fig. 7b a selfed control capsule each seven weeks after pollination. All 7 hybrid capsules were small compared to the self controls with the largest hybrid capsule having a volume only 10% that of the controls. All un-pollinated control pistils abscised without enlarging. The small capsules of hybrid seeds were collected over a 14 day period and the large capsules of selfed seeds were collected at the end of this period as the capsules started to open at the apex. All the hybrid capsules abscised early before the seeds were mature though the largest and last collected contained 96 filled seeds some of which appeared mature. The seeds were sown in March 1989 and 250 filled immature seeds (5% of the total seeds) from the first 6 hybrid capsules collected resulted in 4 seedlings, the largest capsule 10 seedlings. These seedlings were small, lacked vigour and some were chlorotic or deformed with more than two cotyledons. All the hybrid seedlings had glabrous cotyledons and for those that developed foliage leaves the indumentum was juvenile scales with no hairs, Fig. 8. Since this is characteristic of
Vireya
x
Vireya
, the hybridity of these seedlings could not be confirmed from their juvenile indumentum as is the case for
Vireya
x
Azalea
seedlings (Williams et al., 1985). Seeds from the selfed control capsules were 95% filled and of this 66% germinated and the seedlings showed vigour and developed normally.
One year after sowing the seeds 3 healthy hybrid seedlings remain. The largest is 9 cm tall and the top 6 leaves are elliptic (widest near the centre) with a mean ratio of width to length of 0.53 ± 0.01. The selfed control seedlings are 10-12 cm tall and their leaf shape is spatulate (widest near the apex) with mean ratio of width to length of 0.39 ± 0.02. These differences appear sufficient to confirm the hybridity of the largest seedling.
|
|
|
|
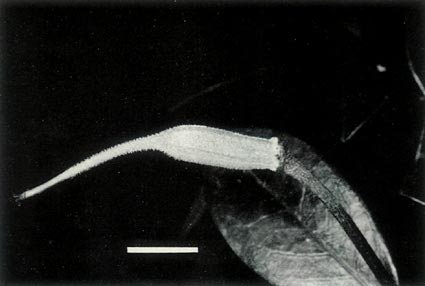
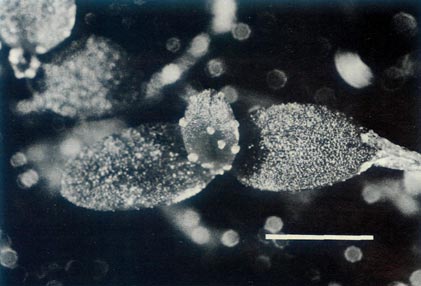
Figure 7: (a) The largest of the 7 capsules of
R. lochiae x R. virgatum 7 weeks after pollination. The dimensional bar represents a length of 1 cm. Photo by J. L. Rouse |
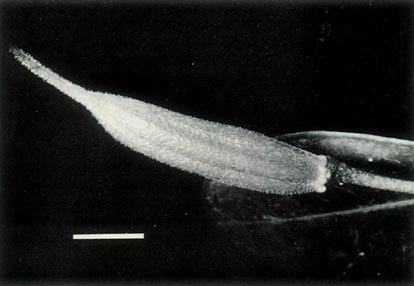
Figure 7: (b) A capsule of
R. lochiae
selfed 7 weeks
after pollination. The hybrid capsule has a volume only 10% that of the selfed capsule and it abscised before properly opening with only some of the seeds mature. Photo by J. L. Rouse |
|
|
Figure 8: The largest of the
R. lochiae
x
R. virgatum seedlings 39 days after sowing the seeds. The cotyledons are glabrous and the first foliage leaf has juvenile scales on the rim and more centrally on the blade. The dimensional bar represents a length of 1 mm. Photo by J. L. Rouse |
Discussion
Investigations into "Barriers to Hybridization in
Rhododendron"
(Williams, Rouse and Knox, in preparation) show that the production of filled seeds from section
Vireya
? x section
Rhododendron
pollinations is unusual, though in many cases the pollen tubes enter the ovary and occasionally the ovules. In 22 trial pollinations the only filled seeds obtained were from the cross
R. retusum
x
R. burmanicum
, though they failed to germinate. Excluding non-tropical Vireyas, reciprocal crosses between species in these two sections appear unlikely to result in seeds as in 9 trials the pollen tubes were arrested in the style and so failed to reach the ovary.
Section
Rhododendron
, however, is "A large section divisible into 27 intricately related subsections" (Cullen, 1980). The species involved form a more heterogeneous group than section
Vireya
which Sleumer (1966)divided into 7 subsections and which more recently Argent et al. (1988) and Argent (1989) have tentatively reduced to 2 subsections,
Vireya
and
Pseudovireya
. So while
R. lochiae
may well be a typical representative species for section
Vireya
,
R. virgatum
is atypical in section
Rhododendron
both in regard to its inflorescence and seed morphology. However the success of the cross
R. lochiae
x
R. virgatum
shows that with time, like the Berlin wall, breeding barriers are breeched and
R.
'Little Pioneer' should encourage other trial pollinations between these two subsections. For success, we suggest the female parent be
Vireya
and that techniques for retaining the capsule on the plant to seed maturity be tried. For entrepreneurial hybridizers, however, who wish to cross an unbreeched barrier, we know of no reports of an authenticated hybrid which span the section
Vireya
- section
Pontica
barrier.
Acknowledgements
John L. Rouse is most grateful for financial support from the Research Foundation of the American Rhododendron Society, the Victorian Branch of the Australian Rhododendron Society and the National Council of the Australian Rhododendron Society. We also wish to thank Miss Julian Champ for her skilled assistance in the preparation of the manuscript.
References
Argent, G. C. G., A. Lamb, A. Phillipps and S. Collertette (1988).
Rhododendrons of Sabah.
Sabah Parks Trustees, Kota Kinabalu, Sabah, Malaysia.
Argent, G.C.G. (1989).
Vireya
taxonomy in field and laboratory. In Smith, J.C. (Ed.)
Proc. 4th Intl. Rhodo. Conf.
, University of Wollongong, Australia. 109-118.
Bailey, F.M. (1900).
The Queensland Flora, Part 3.
924-925.
Blumhardt, O. (1984).
R. lochiae
x
R. virgatum
. "Vireya Vine" newsletter 6:2.
Bulgin, L.W. (1986).
Rhododendron Hybrids, a Compendium by Parent
. Available from author, Ellanhurst Gardens, Rt. 3, Box 223-B, Sherwood, OR 97140, USA.
Cox, P.A. (1985).
The Smaller Rhododendrons
. Batsford Ltd., London.
Cullen, J. (1980). A revision of
Rhododendron
I. Subgenus
Rhododendron
sections
Rhododendron
and
Pognanthum. Notes.
Roy. Bot. Gard., Edinb.39:1-207.
Davidian, H.H. (1982).
The Rhododendron Species
. Volume 1, Lepidotes. Timber Press, Portland, OR.
Feng G. (Ed.) (1988).
Rhododendrons of China
. Volume 1. Science Press, Beijing.
Headlam, A.H. (1976). A growing medium for Malesian Rhododendrons
. Quart. Bull. Am. Rhodo. Soc.
30(2):94-97,103.
Hedegaard, J. (1980). Morphological studies in the genus
Rhododendron
dealing with fruits, seeds and seedlings and their associated hairs, 2 vols. G.E.C. GADS Publishing House, Copenhagen.
Hutchinson, J. (1943).
Rhododendron lochiae
.
Curtis' Bot. Mag.
164: Tab 9651.
Janaki Ammal, E.K. (1950). Polyploidy in the genus
Rhododendron
.
Roy. Hort. Soc. Rhodo. Yearb.
5:92-98.
Janaki Ammal, E.K., I.C. Enock and M. Bridgewater (1950). Chromosome numbers in species of
Rhododendron
.
Roy Hort. Soc. Rhodo. Yearb.
5:78-91.
Kehr, A.E. (1977). Azaleodendron breeding.
Quart. Bull. Am. Rhodo. Soc.
31:226-232.
Mueller, F. von (1887). Description of new Australian plants.
Rhododendron lochiae
.
Victorian Naturalist
3:157-158.
Rouse, J.L. (1984). Pollen storage and
Rhododendron
breeding, p. 185-186. In: E.G. Williams and R.B. Knox (Eds.),
Pollination '84.
School of Botany, University of Melbourne, Australia.
Rouse, J.L. (1985). The propagation of
Rhododendron
section
Vireya
from seed.
Notes.
Roy. Bot. Gard., Edinb. 43:99-115.
Rouse, J.L. (1989).
R. lochiae
x
R. virgatum
. "Vireya Vine" newsletter 22:7.
Rouse, J.L. and E.G. Williams (1989). Style length and hybridization in
Rhododendron
. In: Smith J.C. (Ed.) Proc. 4th Intl. Rhodo. Conf., University of Wollongong, Australia 74-82.
Rouse, J.L, E.G. Williams and R.B. Knox (1986). Floral features related to pollination ecology in
Rhododendron
. In:
Pollination '86, Proc. Symp.
, School of Botany, University of Melbourne, May 1986; 65-69. Reprinted 1987 in
The Rhododendron
(J. Aust. Rhodo. Soc.) 27:4-6.
Rouse, J.L., E.G. Williams and R.B. Knox (1988a). A Vireya azaleodendron in flower.
J. Am. Rhodo. Soc.
42(3): 133-137 and 166-167.
Rouse, J.L., E.G. Williams and R.B. Knox (1988b). The flowering of a Vireya x azalea hybrid.
The Rhododendron
(J. Austral. Rhodo. Soc.) 28(1):12-19.
Sayer, WA. (1888). First ascent of Mount Bellenden-Ker.
Victorian Naturalist
4:37-44.
Sleumer, H.O. (1949). Ein System der Gattung
Rhododendron
. L. Bot. Jahrb. 74:511-553. (English translation p. 1-18. In: J.L. Luteyn and M.E. O'Brien (Eds.),
Contributions Toward a Classification of Rhododendron
. N.Y. Bot. Gard., Bronx, NY.)
Slumer, H.O. (1966). An account of
Rhododendron
in Malesia. Flora Malesiana Ser 1, C.G.J. Van Steenis (Ed.) 6(4):474-668.
Smith, J.C. (1989).
Vireya Rhododendrons
. Australian Rhododendron Society, Olinda, Victoria, Australia.
Stevenson, J.E. (Ed.) (1947).
The Species of Rhododendron
. 2nd Ed. Royal Horticultural Society, London. (1st Ed. 1930).
Synge, P.M. and Platt, J.W.O. (Ed.) (1964).
The Rhododendron Handbook, Part Two, Rhododendron Hybrids
. The Royal Horticultural Society, London.
Williams, E.G. and J.L Rouse (1986). Vireya rhododendron hybrids: an adventure in variety. In:
Rhododendrons with Magnolias and Camellias
. Proc. Roy. Hort. Soc, Lond. 198617:45-62.
Williams, E.G. and J.L. Rouse (1988). Disparate style lengths contribute to isolation of species in
Rhododendron. Austral. |. Bot. 36:
183-191.
Williams, E.G. and J.L. Rouse (1990). Relationships of pollen size, pistil length and pollen tube growth rates in
Rhododendron
, and their influence on hybridization. Sex.
Plant Reprod.
2: (in press).
Williams, E.G., J.L. Rouse and R.B. Knox (1985). Barriers to sexual compatibility in
Rhododendron. Notes
, Roy. Bot. Gard., Edinb. 43:81-98.
Williams, E.G., J.L. Rouse, B.F. Palser and R.B. Knox (1990). Reproductive biology of
Rhododendron
.
Horticultural Reviews
(in press).
Young J. and L.-S. Chong (1980).
Rhododendrons of China
Binford and Mort, OR. Species descriptions and key from Volume III, Iconographia Cormophytorum Sinicorum, Beijing Botanical Research Institute of Academia Sinica, 1974.
Dr. John Rouse is with the School of Physics, The University of Melbourne, Parkville, Victoria, Australia. He has done much research involving the genus Rhododendron . A list of published material is found in the "References" section of this article.
Os Blumhardt is with Koromiko Nursery, Whangarei, New Zealand.