A Cytogenetic Study of Sterile Rhododendron Hybrids
Stephen L. Krebs
David G. Leach Research Station of the Holden Arboretum
Madison, Ohio
Synopsis
Rhododendron breeders usually make interspecific crosses, hybrids between different species, in their quest to combine ornamental traits from each into single cultivars. Occasionally, hybrid plants are produced which are sterile and represent a dead end for further breeding. The occurrence of hybrid sterility increases as the taxonomic distance between the parental species used in the cross increases. In some plant genera, hybrid sterility in wide crosses results from chromosome incompatibilities during the production of sex cells. If chromosomes from different species are too divergent, they fail to pair and divide normally during meiosis, a key event in pollen and egg production. The research presented here examines the possibility of these irregularities causing sterility in three interspecific rhododendron hybrids. The main finding was that the chromosomes from the different species behaved normally during meiosis, an indication that sterility in these hybrids must be caused by other factors.
Introduction
The first chromosome study of rhododendrons was published by Dr. Karl Sax of the Arnold Arboretum in 1930. In a paper titled "Chromosome Stability in the Genus
Rhododendron
," he determined the basic chromosome number
(x=13) as well as the existence of tetraploidy in
R. calendulaceum
and
R. canadense
(13).
His most significant finding, however, was the frequent occurrence of normal meiosis in a wide array of interspecific F
1
hybrids. Chromosomes from taxonomically divergent species exhibited few abnormalities in pairing and disjunction (separation) during meiosis in developing pollen cells. These observations led Dr. Sax to conclude that "there is complete or near complete compatibility of the parental chromosomes in the hybrids, although the parents [Asian and North American rhododendrons] have been separated for millions of years."
This conclusion has been generally substantiated by subsequent research and practical experience. A cytogenetic study of native azalea species found that the frequency of meiotic aberrations and pollen abortion in natural hybrids was low (11), although pollen abnormalities were reported to be more common in deciduous azaleas cultivars (18). In segregating rhododendron populations, however, Mendelian inheritance of isozyme markers was interpreted as evidence that normal meiosis occurs in interspecific hybrids with complex pedigrees (8).
Rhododendron hybridizers have benefited from this broad compatibility among species. Unlike breeders working with other plant genera where strong crossing barriers exist, they are not confined to within species (intraspecific) variability. For over a century they have been successfully recombining traits between species (interspecific). Many of the interspecific hybrids produced are fertile and have been used as parents to produce advanced generation offspring. These historical trends suggest that meiosis is stable in many of these hybrids.
Of course, not all rhododendron hybrids are fertile. This is increasingly true as one moves from intra- to inter-sectional and even wider crosses. Hybrids resulting from crosses between different subgenera, such as azaleodendrons, are often difficult to obtain, and when they do occur they are usually sterile (6). As a breeder interested in transferring traits via wide hybridizations, I have more than a passing curiosity in these infertility barriers. The research presented here represents an attempt to determine the biological mechanisms leading to hybrid sterility in rhododendrons.
A reasonable starting point was to extend Dr. Sax's conclusions to the phenomenon of hybrid sterility. If chromosome "stability" is characteristic of many fertile interspecific hybrids, then chromosome "instability" might be a likely cause of sterility. A chromosomal basis for hybrid sterility has been widely documented in other plant species (2), where structural differences between parental chromosomes interfere with normal pairing and disjunction during meiosis. To test this hypothesis, I examined meiosis in sterile F
1
hybrids resulting from crosses between different subsections of the genus
Rhododendron
.
Methods and Materials
Three lepidote cultivars derived from intersubsectional crosses were used for the study - 'Tow Head' (
R. minus
x
R. ludlowii
), 'Jericho' (
R. keiskei
x
R. minus
'Epoch'), and 'Senegal' (
R. keiskei
x
R. minus
). Although the parental species accessions showed moderate to high levels of pollen fertility, these F
1
hybrids were all judged to be sterile based on high levels (>94%) of pollen abortion (Table 1).
| Table 1 . Rates of pollen abortion in interspecific lepidote hybrids and their parents. | ||
| Clone | Subsection | % Pollen Abortion |
| Parents | ||
| a. R. keiskei Mt. Kuromi form | Triflora | 3.0 |
| b. R. minus Carolinianum Group, Burn's Yellow form | Caroliniana | 58.9 |
| c. R. ludlowii | Uniflora | NA |
| Hybrids | ||
| 'Senegal' (a x b) | 94.4 | |
| 'Jericho' (a x ?)* | 98.0 | |
| 'Tow Head' (b x c) | 98.1 | |
| 'Senegal' siblings (n=5) | 95.3 (mean) | |
| 'Jericho' siblings (n=3) | 97.8 (mean) | |
|
The lepidote F
1
s 'Senegal', Tow Head', and 'Jericho' are registered Leach hybrids. The species accessions 'Mt. Kuromi' (a dwarf form of
R. keiskei
and 'Burn's Yellow' (an ivory form of
R. minus
) are labels used for identification purposes at the Research Station, and are not registered names. The paternal parent of 'Tow Head',
R. ludlowii
, was not available for study.
Percent pollen abortion was estimated by staining pollen with acetocarmine-glycerol (15). A minimum of 100 tetrads per clone were scored for the number of unstained (aborted) monads. Both fresh and one-year-old dessicated pollen stored at -20° C yielded similar scores for this group of plants. * Although 'Jericho' is registered as a hybrid between R. keiskei 'Mt. Kuromi' and 'Epoch' (a tetraploid form of R. minus ),it is probably an open-pollinated seedling from R. keiskei 'Mt. Kuromi'. |
||
Cytogenetic analyses are commonly performed by microscopically examining chromosomes in squashed pollen mother cells (PMCs), cells from immature anthers in which meiosis occurs during the development of mature pollen grains. For the three lepidote hybrids used in this study, meiosis occurred in early October 1995, only a few weeks prior to hard frost at our location. It should be noted that the timing of meiosis varies widely among rhododendrons and is strongly influenced by environmental factors (10, 13, 16).
Flower buds were harvested and fixed for long-term preservation according to the protocols of Vorsa and Ortiz (15) with few modifications. Buds were fixed in a solution of 2:1 (v/v) ethanol: propionic acid. Excised florets were rinsed in 5 exchanges of distilled water and then softened by soaking in a 10% solution of pectinase (Sigma P9179) at room temperature for 48 hours. To observe meiosis, anthers were squashed in a propiono-carmine stain (2% carmine dissolved in a 45 % solution of propionic acid) and viewed under phase contrast optics at 200X and 1000X.
|
|
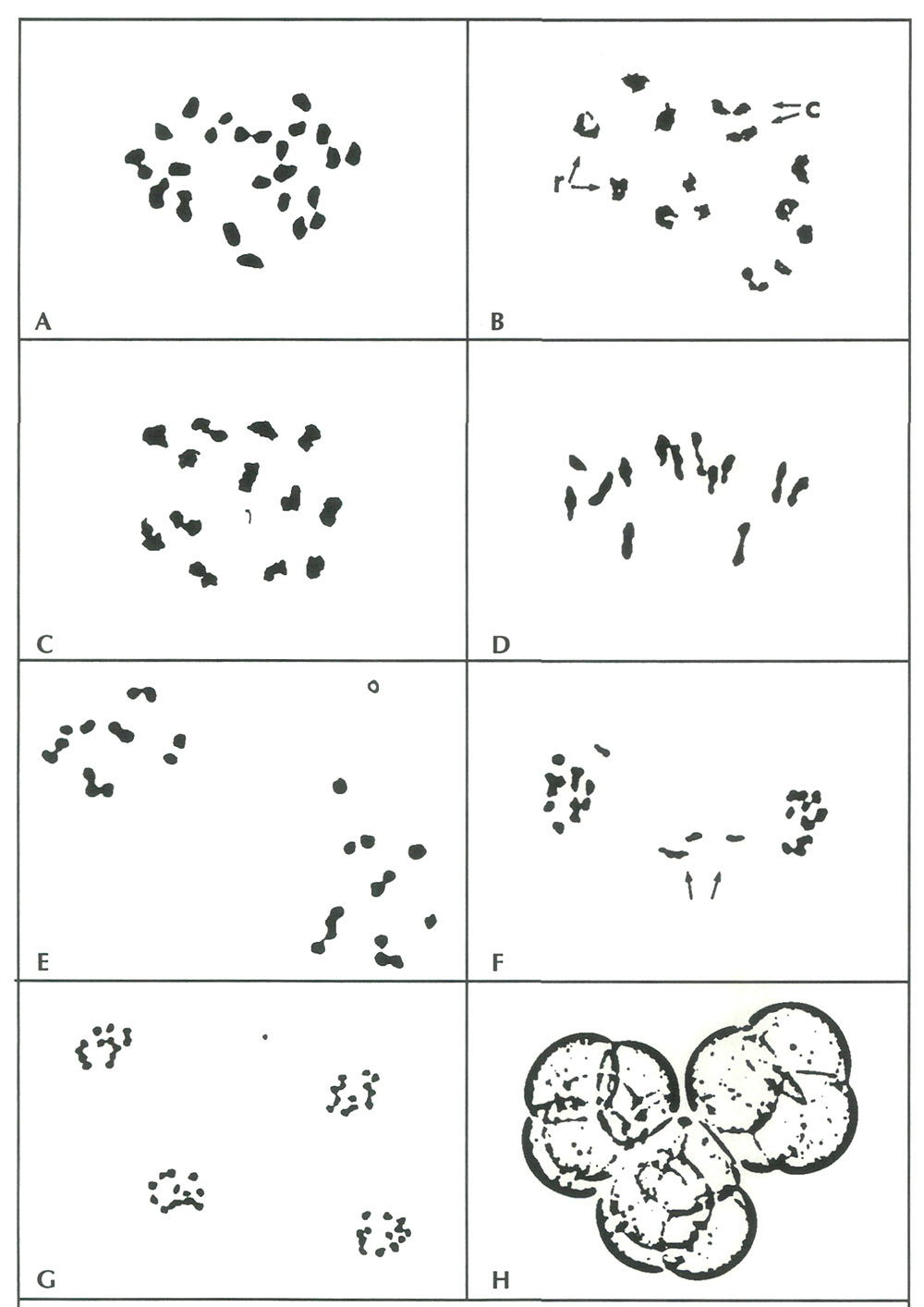
Figure 1. A. Somatic metaphase cell from 'Jericho' with a diploid chromosome number 2x=26 (X 5000). B-G. Stages of
meiosis in rhododendron PMCs. Because all three hybrids exhibited similar patterns of chromosome pairing and disjunction, photographs of each are combined here to portray the key stages in sequence. B. Early diakinesis in 'Tow Head'. Homologous chromosomes have paired to form 13 bivalents in ring [r] and chain [c] configurations (X 3500). C. Late diakinesis in 'Senegal'. The 13 bivalents have condensed further and are about to be aligned along the equatorial plate for the first division (X 4300). D. Metaphase I in 'Tow Head', with 13 bivalents aligned along the divisional plate (X 4000). E. Anaphase I in 'Senegal' showing normal reduction of the diploid nucleus into two haploid daughter cells, each with 13 chromosomes (X 5000). F. Anaphase I in a 'Jericho' PMC with lagging chromosomes (arrows). These configurations were observed in about 5% of the PMCs from 'Jericho' and 'Senegal' (X 3000). G. Four haploid nuclei, each containing the base chromosome number x=13, result from the anaphase II equational division in 'Jericho' (X2500). This tetrad is the precursor to the mature pollen grain shown in H (X 750). |
Results and Discussion
Lack of pollen staining indicated that nearly all the male gametes produced by 'Tow Head', 'Jericho', and 'Senegal' were aborted even though their parents had moderate to high levels of pollen viability (Table 1). Siblings of 'Jericho' and 'Senegal' also showed high rates of pollen abortion (> 95%), suggesting that hybrid sterility was a typical result from these particular subsectional crosses.
Pollen staining often overestimates viability when compared to
in vitro
and
in vivo
germination tests (14, 17), and so it is reasonable to conclude that these clones have extremely low levels of male fertility. Although conclusive data are lacking, it is quite likely that these clones are female sterile as well. One cultivar, 'Tow Head', has failed as a seed parent in several controlled crosses at the Leach Research Station, and none of the three cultivars has set open-pollinated seed capsules in the past two years.
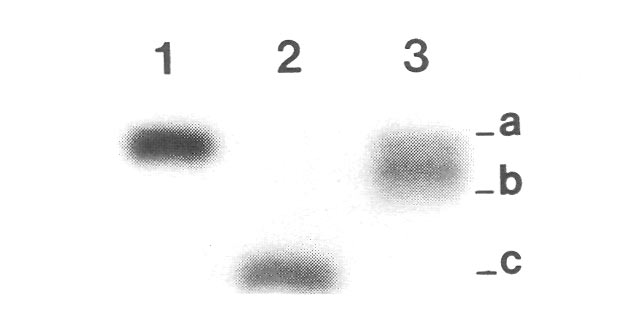
The cultivar 'Jericho' is registered as a hybrid between diploid
R. keiskei
Mt Kuromi form, and 'Epoch', the tetraploid form of
R. minus
synthesized by Dr. Kehr (5). I included it in this study because crosses between diploids and tetraploids often generate sterile triploid progeny. However, chromosome counts in both mitotic and meiotic cells indicated that 'Jericho' is a diploid (Fig. 1A, 1G). Hybrid verification using isozyme markers (enzyme variants) further demonstrated that 'Jericho' carries genes attributable to the seed parent but not to 'Epoch' (Fig. 2). There is therefore little doubt that 'Jericho' results from an open-pollinated seedling from
R. keiskei
. The real father may be one of the numerous diploid
R. minus
clones that are in close proximity to, and bloom concurrently with,
R. keiskei
at the Research Station. If this is the case, then 'Jericho' is, for the purposes of this study, fairly similar to 'Senegal' in its genetic composition.
|
|
Figure 2. Photograph of enzyme banding patterns (isozymes) produced by genes encoding phosphogluconate
dehydrogenase (PGD) in three different individuals: lane 1 = R. keiskei , Mt. Kuromi form, lane 2 = R. minus 'Epoch', lane 3 = 'Jericho', the registered hybrid of R. keiskei , Mt. Kuromi form x 'Epoch'. Protocols for visualizing and interpreting isoymes followed earlier reports (7, 8). Letters on the right refer to different alleles found in these individuals at the Pgd-1 gene. For this dimeric enzyme, single bands represent homozygotes and triple bands indicate heterozygotes. The seed parent R. keiskei , Mt. Kuromi form, has a homozygous aa genotype, and the putative pollen parent 'Epoch', a tetraploid, is homozygous cccc . A true hybrid is expected to have a triploid acc heterozygous genotype. The cultivar 'Jericho' is heterozygous (the three bands are quite close together and almost appear as a single wide band), but it is an ab heterozygote - it carries the a allele from R. keiskei and a b allele found in neither parent. This means that 'Epoch' cannot be a parent of 'Jericho', and strongly suggests that 'Jericho' is an open-pollinated seedling collected from R. keiskei . These results were confirmed at two other enzyme loci as well (data not shown). |
Stages of meiosis from early prophase through late anaphase I, which encompass the reduction division (separation of homologous chromosomes), were observed in buds from all three cultivars. Equational cell divisions (separation of sister chromatids) at anaphase II were seen only in 'Jericho' and 'Tow Head' PMCs. Apparently, the flower buds sampled from 'Senegal' were picked a little prematurely to be able to observe the later meiotic stages. Nonetheless, configurations at diakinesis (early pairing of chromosomes) and anaphase I (disjunction of paired chromosomes) are adequate for determining whether chromosomes from the parental species are compatible or not.
Meiosis was predominantly normal in PMCs from all three hybrids. At diakinesis, the 26 chromosomes were organized throughout the nuclei into 13 pairs (bivalents), either as ring bivalents or as rod bivalents (Fig 1B, 1C ). This meiotic arrangement would be expected in any diploid organism with a base chromosome number of 13. These paired configurations were subsequently maintained at metaphase I, where the chromosomes were aligned along a divisional plane in the cell nucleus (Fig 1D).
No univalents (unpaired chromosomes) were observed at diakinesis or metaphase I. Univalents are usually indicative of chromosome divergence and incompatibility, and because they separate randomly during the reduction division of meiosis, they can result in daughter cells with less than the full haploid complement of chromosomes. Rhododendron pollen cells lacking chromosomes (x < 13) also lack key genetic material for survival and are usually aborted.
Occasional irregularities were observed in some hybrids during anaphase I, but the majority of PMCs displayed normal reduction division of diploid cells (2x=26) into two haploid cells with 13 chromosomes (Fig 1E ). The irregularities were all of one type - lagging chromosomes which were slower to move towards the divisional "poles" and thus appeared in the middle of cells between the two main dividing groups of chromosomes (Fig 1F). These lagging chromosomes are also often excluded from daughter nuclei, resulting in non-viable, aborted pollen cells. In the three hybrids studied here, however, the "laggards" cannot account fully for hybrid sterility because they occurred at a frequency (5% of PMCs in 'Jericho' and 'Senegal') far below the 95% pollen abortion rate.
In 'Jericho' and 'Tow Head', numerous observations were made on PMCs undergoing the second (equational) division. The chromosome configurations and numbers in these cells were also normal - by late anaphase II, pollen tetrads invariably contained the expected 13 chromosomes per monad (Fig. 1G).
High rates of pollen sterility in these interspecific hybrids are therefore not attributable to meiotic irregularities. These results suggest that Sax's concept of chromosome compatibility can be extended to sterile as well as fertile rhododendron hybrids. Even though pairing and disjunction of chromosomes from morphologically divergent species is normal, the pollen may ultimately be unviable.
Other nuclear or cytoplasmic factors could account for sterility in these individuals. Cytoplasmic hybrid sterility occurs when there is discordance between the cytoplasm of one species (maternally inherited) and nuclear genes from the pollen species. Genie hybrid sterility results from lethal interactions between nuclear genes from both parents. For example, genes from
R. minus
and
R. keiskei
that are responsible for pollen development after meiosis may not function properly when combined in the hybrid nucleus of 'Senegal'.
Future experiments to test these genetic hypotheses would initially require reciprocal crosses in which each species is used as both male and female parent (e.g.,
keiskei
x
minus
and
minus
x
keiskei
). If cytoplasmic factors are involved, the direction of the cross will affect fertility levels in the progeny because each species used as a seed parent contributes a different cytoplasm. If no reciprocal differences in fertility are observed among the progeny, then lethal nuclear genes are most likely involved. At the present time, experimental populations which could address these issues are not available at the Research Station.
The apparent lack of chromosomal divergence stands in stark contrast to the morphological and adaptive changes that have occurred among
Rhododendron
species over evolutionary time. Chromosomal divergence among species is usually attributable to structural changes such as inversions, translocations, and duplications - physical alterations in the arrangement of chromosome segments that interfere with homologous pairing. One possible reason for conserved structure in rhododendrons is the small size of the chromosomes, which appear to be in the 0.5 - 2 micron range based on my observations and those made in
Ledum
(9). The relatively short chromosome arms could physically restrict wholesale exchanges of chromosome segments and maintain a fairly uniform structure among species.
Hybrid sterility is a natural crossing barrier which plant breeders attempt to overcome in order to recombine genes from different species. When F
1
sterility is caused by chromosomal incompatibilities, fertility can often be restored by doubling the chromosome number through colchicine treatments (1). This allows for normal bivalent pairing of homologous chromosomes from the same species in the synthesized tetraploid. However, the type of barrier presented by genic hybrid sterility could be insurmountable because chromosome doubling may not alter lethal interactions among the genes involved. In all likelihood, the sterility observed in 'Jericho', 'Senegal', and Tow Head' represents a breeding barrier that will be extremely difficult, if not impossible to circumvent.
It remains to be seen whether observations of chromosome stability at the subsectional level can be extended to the outer limits of rhododendron hybridization. There are several reports of progeny derived from crosses among subgenera, and many of these wide hybrids are infertile (3, 4, 6, 12). A cytogenetic study of PMCs from these plants could ultimately determine whether any significant chromosome divergence has taken place in the genus
Rhododendron
, or whether, as suggested by this research, the causes of hybrid sterility lie outside the issue of chromosome incompatibility.
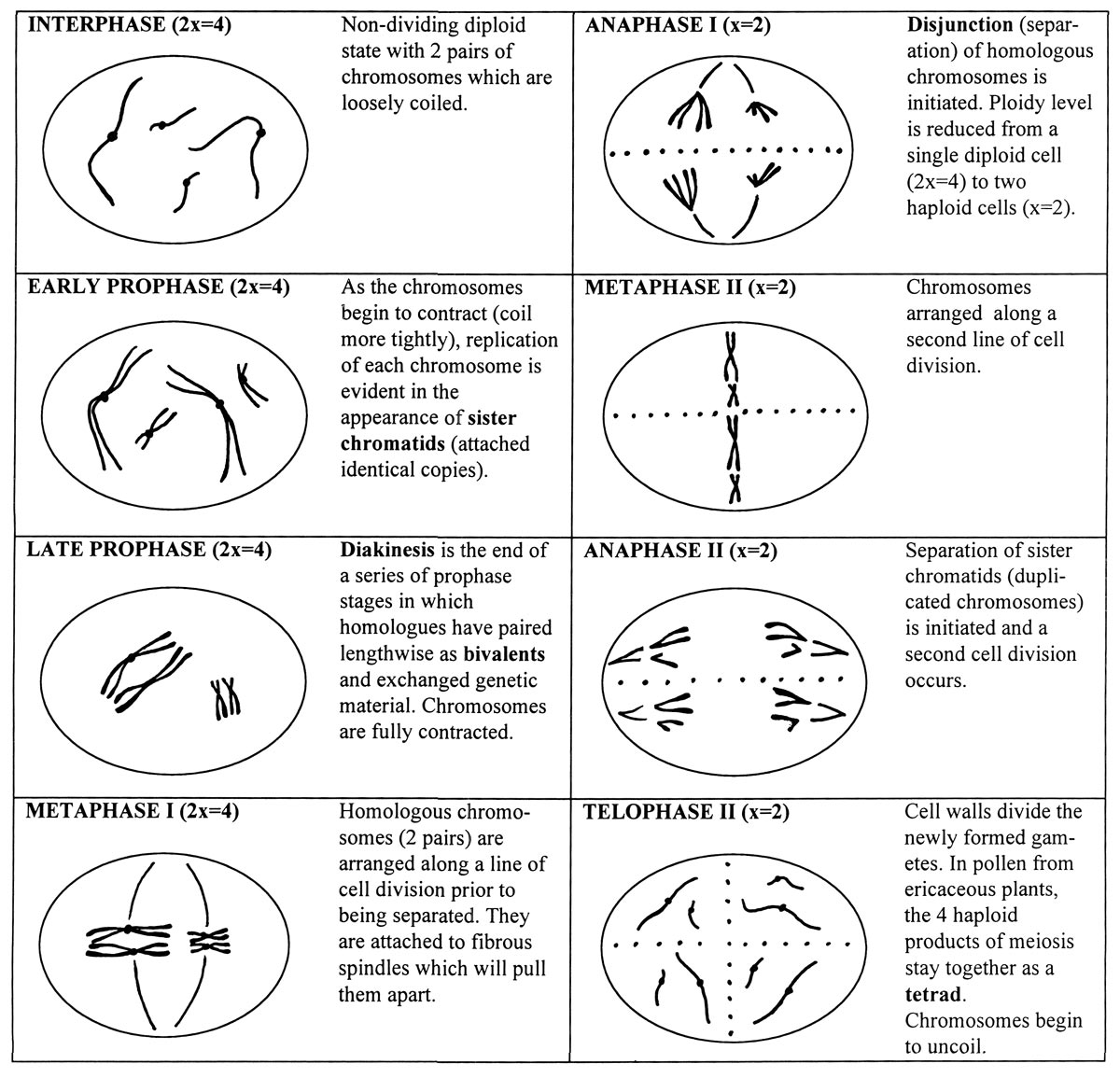
Stages of Meiosis
Cells of higher organisms possess a basic chromosome number or ploidy (x), the smallest number of chromosomes in a chromosome set. Multiple sets of chromosomes are usually required in order for cells to function normally during the growth and development of plants and animals. Diploid organisms have 2 chromosome sets (2x), while polyploids have 3 or more sets - for example triploids (3x) and tetraploids (4x). Each chromosome in a set is distinct in physical and genetic structure. In diploids, identical chromosomes from each set are called homologous pairs or homologues.
When organisms reproduce, they form specialized sex cells or gametes in which the normal chromosome number is halved by the process of meiosis. The reduction in ploidy is accomplished by cell divisions. In the case of diploid plants, pollen and egg cells are haploid and contain a single set of chromosomes. Upon fertilization, the diploid condition is restored to the embryo and the process is repeated in subsequent generations.
Most of the stages of meiosis are outlined below in a hypothetical diploid organism having a basic chromosome number x = 2. The chromosomes are distinguishable by length; one is short, the other long. The fundamental events in meiosis are 1) pairing of homologous of chromosomes 2) physical exchange of genetic material and 3) subsequent separation of homologues and reduction of cell ploidy level by one half.
|
| Stages of Meiosis |
Literature Cited
1. Briggs, F.N.; Knowles , P.F. Introduction to plant breeding. Rheinhold Publishing; 1967.
2. Dobzhansky, T. Genetics and the origin of species. Columbia Univ. Press, New York; 1937.
3. Heyting, J. Hybrids between elepidote and lepidote rhododendrons. Q. Bull. Amer. Rhod. Soc. 24: 97-98; 1970.
4. Jaynes, R.A. F
1
crosses of evergreen and deciduous azaleas and other wide crosses of rhododendron. Q. Bull. Amer. Rhod. Soc. 30: 44-48; 1976.
5. Kehr, A. A tetraploid
Rhododendron carolinianum
. Q. Bull. Amer. Rhod. Soc. 25: 4-7; 1971.
6. Kehr, A. Azaleodendron breeding. Q. Bull. Amer. Rhod. Soc. 31: 226-232; 1977.
7. Krebs, S.L Enzyme fingerprinting of rhododendrons. J. Amer. Rhod. Soc. 49: 210-215; 1995.
8. Krebs, S.L. Normal segregation of allozyme markers in complex rhododendron hybrids. J. Heredity 87: 131-135; 1996.
9. Lantai, K.; Kihlman, B. The chromosome numbers of
Ledum palustre
ssp.
decumbens
and some related taxa. Hereditas 122: 181-184; 1995.
10. Leach, D.G.; Stowe, W.C.; Fink, C.V.M. Seasonal changes in anther development of cold hardy rhododendrons. J. Amer. Rhod. Soc. 43: 128-132; 1989.
11. Li, H. Chromosome studies in the azaleas of eastern North America. Amer. J. Bot. 44: 8-14; 1957.
12. Martin, C. Breeding azaleodendrons. Q. Bull. Amer. Rhod. Soc. 24: 39-41; 1970.
13. Sax, K. Chromosomal stability in the genus
Rhododendron
. Amer. J. Bot. 17: 247-251; 1930.
14. Stanley, R.G.; Linskens, H.F. Pollen: biology, biochemistry, management. Springer, Berlin; 1974.
15. Vorsa, N.; Ortiz, R. Cytology of 2n pollen formation in a blueberry aneuploid (2n=4X + 9=57). J. Heredity. 83: 346-349; 1992.
16. Widrlechner, M.P.; Pellett, H.M.; Ascher, P.D. The timing of microsporogenesis in deciduous azaleas. J. Amer. Rhod. Soc. 37: 91-94; 1983.
17. Widrlechner, M.P.; Pellet, H.M.; Ascher, P.D.; Fuhrman, S.C.
In vivo
pollen germination and vital staining in deciduous azaleas. HortScience 18: 86-88; 1983.
18. Widrlechner, M.P.; Pellett, H.M. Notes on the pollen of deciduous azalea cultivars. J. Amer. Rhod. Soc. 37: 210-212; 1983.
Dr. Krebs authored the article "Enzyme Fingerprinting of Rhododendron Cultivars" appearing in the Fall 1995 issue of the Journal.