Osmosis and Plant Nutrition
Michael Hammer
Sassafras, Victoria, Australia
Reprinted from the The Rhododendron, Vol. 40, 2000, the journal of the Australian Rhododendron Society.
What is Osmosis?
Imagine we take a container and fill it with water. The water consists of many tiny molecules in constant movement. As these molecules move around they collide with the walls of the container and bounce back, millions upon millions of collisions per second. Each collision exerts a tiny push on the wall and the overall result of all these collisions is a net force on the walls of the container which we call "pressure." The pressure is simply a measure of the number of collisions per square metre per second.
Next, imagine we put a barrier down the middle of the container (see Figure 1) so that there is water on both sides of it. Further, we make this barrier out of a material which has tiny holes in it. These holes are far too small to see, but they are large enough to allow a water molecule to fit through. The water molecules will collide with this barrier just as they collide with all the other wall surfaces. Most of the time they hit a solid part of the barrier and bounce back, but occasionally they will strike the barrier where there is a hole and pass through it. Thus, referring to Figure 1, some water molecules from side A will end up moving into side B and vice versa.
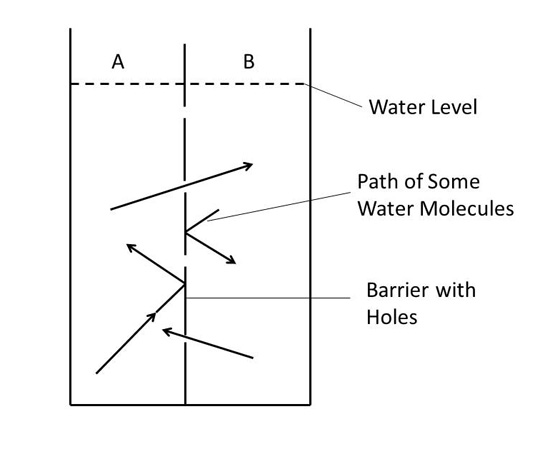
|
| Figure 1. Barrier with water on both sides. |
Now imagine we dissolve some salt into side A of our container. Salt molecules are larger than water molecules, and if the holes in our barrier are of the right size they can be big enough for the water molecules to pass through but too small to allow the salt molecules through. Since the pressure on both sides of the barrier is the same, the total number of collisions per second on both sides of the barrier will be the same, but, on side B, all collisions are due to water molecules (which can fit through the holes), whereas on side A some are due to salt molecules (which cannot fit through the holes). Fewer water molecules strike the barrier per second on side A than on side B and thus fewer water molecules will pass through the barrier from side A to B than from B to A. There is a net movement of water from the side of low salt concentration to the side of high salt concentration.
This effect is called "osmosis," and the sort of barrier we've just talked about, with holes large enough for some molecules and too small for other molecules, is called a "semi-permeable membrane." Of course we do not have to use salt; any substance which dissolves and has large molecules will cause the same effect.
Osmotic Pressure
The flow of water from one side of the membrane to the other occurs simply because there are more collisions per second of water on one side of the membrane than on the other. The flow will continue until the number of collisions per second of water molecules becomes the same on both sides of the membrane. This can occur in two ways.
Firstly, it can occur if the migration of water causes the concentration of dissolved solids to equalize on both sides of the membrane. This could occur if there was material dissolved in the liquid on both sides of the membrane but at different concentrations.
Secondly, it can occur if the migration of water raises the physical pressure on the more concentrated side of the membrane. More pressure means more collisions per second, and if the pressure is raised enough, the number of collisions due to the water component will balance. This forms a convenient way to measure the osmotic strength of a solution and leads to the expression "osmotic pressure" as a measure of the osmotic strength of a particular solution.
Imagine we have a semi-permeable membrane in the form of a closed sack with an aqueous solution of salts inside it. If we place this sack in a solution with more dissolved solids (greater osmotic pressure), water will be drawn out of the sack and it will start to collapse. If we place the sack in a solution with less dissolved solids (lower osmotic pressure) water will flow into the sack and it will start to swell.
So why is all this relevant to us as gardeners? Well, it turns out that many membranes in nature are semi-permeable. In particular, cell walls are semi-permeable membranes. We may not realize it but we are constantly experiencing the effects of osmosis in everyday life. Here are some everyday examples.
Some Examples of Osmosis
You cut yourself, put seawater on it and it hurts. The osmotic pressure of seawater is much higher than the inside of your cells (seawater is about 2-3% salt); the exposed cells start to lose water and collapse. Put fresh water on it and it also hurts; the osmotic pressure of the fresh water is too low, and the cells gain water and start to swell. If you bathe the cut in water with 0.9% salt (saline), however, it doesn't hurt at all: the osmotic pressure of the saline is just right. Try it next time you want to wash a cut or graze. Add 9 grams of salt (about 2 level teaspoons or 1 heaped teaspoon) to a litre of boiled water. You should find that it is much less painful. Of course, be careful - don't add too much salt or the osmotic pressure will become too high and it will hurt again.
In the past people used to salt meat to keep it from spoiling. Why? The bacteria that attack meat do not have an impermeable skin; they have cell membranes that are semi-permeable. The osmotic pressure of the salt is so high it sucks the water out of the bacteria cells and kills them. By the way, that is the basis for the belief that bathing a wound in seawater helped to fight infection.
Salt is not the only chemical that raises osmotic pressure. Any soluble molecule large enough to be blocked by the holes in the membrane has a similar effect. One very important class of molecule with a similar effect are the sugars. Bacteria use sugar for food just as we do. Despite this, a strong enough sugar solution will raise the osmotic pressure high enough to kill bacteria and thus prevent spoilage - that's why jams and honey keep well without refrigeration. If you don't believe me, try diluting honey with some water and leave it out for a few days. Compare its lasting qualities with undiluted honey.
The membranes of plant cells are also semi-permeable. There are some simple experiments you can do to show this. Put some raw cucumber slices in a bowl of fresh water and others in a bowl with saturated salt water. The slices in fresh water swell up and become very turgid. The ones in salt water collapse and become completely limp.
Take a half a raw potato, scoop a recess in the cut face and put in a spoonful of salt or sugar. Leave it for an hour or so and you will find the recess filled with liquid, while the potato around the liquid has gone soft and spongy. Some of the salt or sugar dissolves in the little bit of water around the cut face and the high osmotic pressure of this solution draws out more water from the potato cells. Try it with a cooked potato and nothing happens. Why? Cooking destroys the cell membranes so that osmosis can no longer occur.
Effect of Osmosis on Plants' Collection of Water
Root hairs on plants, like other cells, are also semi-permeable. Water gets through readily but dissolved nutrients cannot. Plants, in fact, rely on an osmotic pressure gradient in order to collect water. The concentration of dissolved solids, and thus the osmotic pressure, rises continuously from the soil around the roots to the central water conducting core of the root (called the xylem) and this causes water to flow into the plant. Remember we said osmosis can result in a physical pressure difference across the membrane - this means that the physical pressure is higher in the core of the root than in the soil around the plant. On cool mornings especially, when the soil is damp, you can sometimes see drops of water all round the edges of the leaves on some plants. This arises because the osmotic pressure gradient has forced so much water into the plant it flows out through the ends of the veins at the edges of the leaves and collects as droplets. Botanists call this process "guttation."
By the way, as an aside, did you know that plants cool themselves by evaporating water the same way we do when we perspire? This partly explains why plants burn much more easily when they dry out. Without enough water to evaporate they can't cool themselves adequately and the leaves overheat and die.
The primary molecule raising the osmotic pressure inside plant roots is sugar - manufactured in the leaves and transported down in the phloem tissue to the roots. Plants to some degree can control the osmotic pressure inside their roots. This is done by converting sugar to starch or vice versa. Starch is only sparingly soluble, so it does not contribute much to osmotic pressure. If a plant wants to reduce its osmotic pressure it converts some sugar to starch. To raise the osmotic pressure it can convert some starch back into sugar.
Root hairs do not just collect water for the plant; they also collect nutrients by a separate process called "active transport." For this process to work, however, the nutrients have to be dissolved in water. Nutrients in an insoluble form cannot be absorbed by the plant. For example, you can't address an iron deficiency for an azalea by putting some iron filings around the plant. The iron may be there, but it is not in soluble form, so the plant can't take it up. And herein lies a paradox, exactly the same as for the bacteria in honey. Because the nutrients are soluble in water they also raise the osmotic pressure outside the root hairs. A higher nutrient level means more food but it also makes it harder for the plant to collect water. If the nutrient concentration becomes too high the osmotic pressure outside the roots becomes greater than inside the roots. When that happens the flow of liquid reverses. Instead of the plant taking up water and nutrients it can't take up anything. Instead it starts to lose water into the surrounding soil. The plant dehydrates, the leaves are starved of water, they dry out, die and go brown around the edges. We say the plant is being burnt. If the situation lasts too long the plant dies.
Controlling Osmotic Pressure Around Plant Roots
How can the osmotic pressure get to be higher in the soil than in the plant? Firstly and most obviously, you put too much fertilizer around a plant. Less obviously, you fertilize a plant when the soil is very wet, the fertilizer is well diluted and at a reasonable concentration for the plant. Then along comes a dry spell and the soil around the plant starts to dry out. Water is lost but the nutrients cannot evaporate; they stay in the soil and the concentration rises and rises. Eventually it gets so high the osmotic pressure reverses and goodbye plant. Another problem is especially relevant to pot plants. Every time you fertilize you add more nutrients to the pot. Normally you add far more nutrients than the plant can actually use. The excess cannot escape and builds up around the plant roots. Eventually the level reaches toxic levels, and as mentioned already this is exacerbated when the mix in the soil dries out a bit. To avoid this, one is told to periodically deep soak pot plants to wash out the excess nutrients.
But another issue needs to be considered as well. What is the osmotic pressure inside the plant? If this is high enough the plant can cope with a higher concentration of nutrients in the soil. Remember sugar was the main molecule raising osmotic pressure inside plants. The osmotic pressure is likely to be highest when there is a lot of sugar around and this occurs when the plant is producing the greatest amount of sugar - and when is that? When it is most active, when it is growing most rapidly. Conversely, the sugar level is likely to be lowest when the plant is dormant. Hence the advice to fertilize plants when they are growing rapidly and the caution to not fertilize when the plant is dormant.
What about cuttings? The greatest problem for a cutting is loss of water. Further, the cutting is using up food reserves to produce new roots. Sugar levels are likely to be pretty low and this means the osmotic pressure inside the plant will also be low. A bad combination. The last thing a cutting in that position can cope with is high osmotic pressure outside the fledgling roots. We want to make the osmotic pressure outside the cutting as low as possible. Fertilizer for a cutting is like poison. It is not that the cutting can't use the nutrients. That is irrelevant; it would only mean the fertilizer was wasted. The problem is that the nutrients raise the osmotic pressure and dehydrate the cutting. In fact we probably should be thoroughly washing our mix to remove every trace of dissolved solids to get the osmotic pressure as low as we possibly can.
Regulating the Nutrient Level Around Plants
One of the challenges for us as gardeners is to regulate the nutrient level around our plants. Plants can cope with considerable variation in the level of nutrients around the roots, but they do better if the level is more stable. That's why the comment is made that it is better to fertilize more often with very weak fertilizer than it is to use stronger fertilizer occasionally.
Let's look a bit at ways in which nutrient levels can be stabilized around plants. The key here is that nutrients are available to plants and affect the osmotic pressure only if they are in solution. Nutrients not in solution are completely inert as far as the plant is concerned. You know, what would be really nice would be to have some mechanism which stored nutrients in the soil in an insoluble form and slowly converted them to a soluble form at a rate which keeps a constant level around the plant. You often hear comments that organic fertilizers - compost, manures, etc. - are far better than chemical fertilizers. Environmentalists and "greenies" often wax so lyrical it seems as though the nutrients from organic fertilizers are good and healthy while the nutrients in chemical fertilizers are evil and poisonous. That of course is utter rubbish; a potassium ion is a potassium ion whatever the source. Organic fertilizers do, however, have a major advantage. The nutrients in chemical fertilizers are in a readily soluble form. Very shortly after the fertilizer is applied to the soil, the nutrients dissolve, raising the nutrient level and osmotic pressure. The nutrients in organic fertilizers, however, are often locked up in complex organic compounds and do not dissolve readily. When they are applied to the soil it requires the action of microbes in the soil to break down these organic compounds and thereby release the nutrients to dissolve in the soil water. Thus organic fertilizers provide a slow steady nutrient release. In more recent years, inorganic fertilizers have become available which can at least partly match this action. Fertilizer granules are coated with a polymer which prevents the fertilizer dissolving all at once. Instead the nutrient material slowly leaches through the polymer barrier. Depending on the thickness and composition of this barrier, the leaching process can take three, six or nine months. Several proprietary brands are available, of which the best known is probably Osmocote (the name probably comes from a contraction of "osmotic coating").
There is another advantage of organic fertilizers. They leave a residue of partly decayed organic matter in the soil called "humus." This humus changes the way in which soil particles stick together, and also has the property of binding and trapping both water and nutrients. Nutrients can continuously attach and de-attach themselves to humus particles (called an "equilibrium reaction"). When the nutrient concentration in the soil is high, the rate of attachment exceeds the rate of separation. The net effect is that some of the nutrients bind to humus particles and are effectively removed from solution. When the dissolved nutrient level falls, the equilibrium swings the other way and the attached particles go back into solution. In short, the humus acts to stabilize the dissolved nutrient level in the soil water - exactly what we discussed just before. Chemists call this process "buffering." Thus organic fertilizers provide a buffered source of nutrients whereas chemical fertilizers are an unbuffered source.
Humus is not the only thing which can do this. Clay particles such as felspars, silicates, etc., are chemically active materials. Nutrients can adhere and detach from them, just as happens with humus. Again, when nutrient levels are high, attachment predominates and the nutrients are removed from solution but still bound in the soil so that they are not washed away. When dissolved levels fall again the bound nutrients detach, raising the dissolved levels again. By contrast, sand is silicon dioxide which is chemically inert. Nutrients cannot attach to sand particles. As a result, the nutrient level fluctuates much more in sandy soils than in clay and nutrients are much more easily washed away and lost. Clay soils may have problems with poor aeration, compaction and water logging but they are generally more fertile than sands.
Nutrient Availability Versus pH
Osmosis explains how plants absorb water from the soil but it does not account for the way in which a plant collects nutrients. In general, collection of nutrients is a more complex active process (a pumping process which requires the plant to expend energy). It is also a process that varies very greatly from one type of plant to another. In general, if a plant species is growing in an environment where a particular nutrient is very scarce it evolves very efficient ways of collecting that nutrient. Conversely, if the plant grows in an environment where a particular nutrient is very plentiful the collection efficiency for that nutrient can be expected to be very low. Indeed, if the nutrient is normally present in excessive amounts the plant may even develop mechanisms to reject that particular nutrient. A simple example of that is when plants colonize the tidal margins such as saltwater mangroves. In these locations the sodium concentrations (at least) are much higher than the plant can possibly use and these plants need to develop mechanisms to selectively excrete the excess sodium.
If a plant has developed in a region where a particular nutrient is very low and is suddenly placed in an environment where there is a large amount of the nutrient, its super-efficient collection mechanism means that it will collect far too much of the nutrient - possibly a toxic level. Such a plant has no means of getting rid of the excess, because it evolved in an environment where such a mechanism was not necessary. This is, for example, the situation for many Australian natives with regard to phosphorous. This does not mean that Australian natives use less phosphorous for growth. It only means they are more efficient at collecting it and therefore require lower levels of this element in the soil.
Conversely, if a plant species evolved in a region where a nutrient was very plentiful, and it is placed in a new environment where that nutrient is much less plentiful, then the plant may suffer a deficiency simply because it has not developed efficient mechanisms for collecting that nutrient. A good example of that is the Rhododendron genus with respect to iron. Rhododendrons are so inefficient at collecting iron they can suffer chlorosis at available iron levels which would be more than adequate for, say, vegetables.
Remember, a nutrient is only available if it is in solution. It is quite possible for plenty of the nutrient to be present yet not in solution - it may be present as an insoluble salt. A major factor influencing this is the pH of the soil. You can easily show this with a simple experiment. Put some ferrous sulphate (sulphate of iron) in water and shake it up. The ferrous sulphate dissolves to form a clear green solution. Now add some washing soda (sodium carbonate) or some caustic soda (sodium hydroxide) and shake again; either of these materials will make the water alkaline. Immediately a dirty brown precipitate forms and the green colour disappears. That brown precipitate contains the iron converted to an insoluble form, a form which is useless to plants. That is why adding ferrous sulphate to alkaline soil makes very little difference to rhododendrons, as the ferrous sulphate is immediately converted to insoluble form.
One needs to change the soil pH, not the total iron level. An alternative solution is to add the iron in a form which is not readily rendered insoluble - iron chelates (iron in this form is unfortunately relatively expensive).
This interdependence between availability and pH applies to most soil nutrients. It can be shown in diagram form (Figure 2). The issue of nutrient availability is in fact the main reason behind plant sensitivity to pH. Thus vegetables, which grow quickly and need large amounts of the major nutrients nitrogen, phosphorus and potassium, grow best at a pH between about 6.5-7.5. Plants that have trouble collecting enough iron (azaleas, rhododendrons, etc.) grow best at a pH between about 5 and 6.
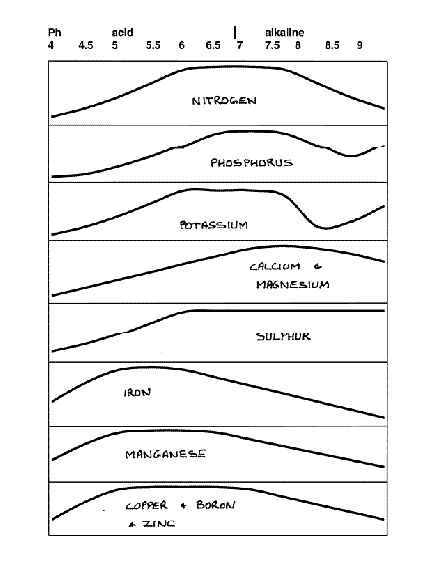
|
|
Figure 2. Nutrient availability verses pH. The higher the graph,
the more nutrients are available. |
Controlling pH
If we find our soil is too alkaline (pH too high) can't we lower it by adding an acid? For example, could we add some hydrochloric acid (brick cleaning acid or pool acid)? Conversely, if the pH is too low can we add some sodium hydroxide - caustic soda? The simple answer is no, that will not work - it will either do nothing or it will kill your plants. The problem is a little bit similar to the problem with chemical fertilizers that fully dissolve as soon as applied. Imagine I take 1 litre of distilled water which will be pH 7 - neutral. If I add one drop of hydrochloric acid the pH will fall from 7 to 3. Well, okay, we know hydrochloric acid is a very strong acid, so maybe I just used too much. If one drop per litre gave pH 3 then one drop per 100 litres should give pH 5. True, it will, but then one drop of similar strength caustic soda will take you back to pH 7 and two drops would take you to pH 9! You may be able to get the water to pH 5 using hydrochloric acid but you could never keep it there. The problem is that all the acid is fully expressed. This firstly means that its initial effect is far too severe, and secondly there is nothing in reserve to keep the pH stable against external factors that could change it. So it swings up and down like a yo-yo. Just as with fertilizers, we come up against the concept of buffering. We want the pH not only to be correct but also to stay correct despite perturbing factors. In practice all soils have natural buffering; they all resist changes to pH to some degree. The smaller the amount of buffering the easier it is to change the pH, but the more readily the pH will drift away from the desired level, i.e., the less stable the soil. The greater the buffering the more stable the soil but the harder it is to change the pH. That, by the way, is why adding a bit of hydrochloric acid to the soil would probably have no effect; the natural buffers in the soil would neutralize it without any significant change to the overall pH.
Just as we discussed before, and for much the same reasons, sands exhibit a low level of buffering, whereas clays and humus rich soils exhibit a high level of buffering. We need more material to change the pH of clay soils than we need for sandy soils. Indeed, in some cases the soil can be so well buffered that it is almost impossible to make any meaningful change to pH. This is especially the case for limestone rich soils which are naturally alkaline.
If we want to make any meaningful change to soil pH we need to use materials which exert a strong buffered effect. They may not push the pH very far, but they exert a lot of force to maintain the change despite other influences. Just as for fertilizers, this implies materials which are expressed slowly. Materials expressed quickly may make a short-term change to the pH but it will tend to drift back as the material added becomes exhausted.
There is a very convenient material we can use to make soil more alkaline (raise the pH) and that is lime. It is rapid in initial action and the effect lasts for quite a long time. Unfortunately the word lime is used for two distinctly different chemicals. Slaked lime or "builders lime" (sold under the name Limil around here) is calcium hydroxide. By contrast, "garden lime" is calcium carbonate. Another similar material often recommended is dolomite, which is a mixture of calcium carbonate and magnesium carbonate. Calcium hydroxide - builders lime - is much more strongly alkaline, and therefore more likely to burn plants and even unprotected skin. Therefore, in principle, calcium carbonate is a better choice. In practice, calcium hydroxide rapidly absorbs carbon dioxide from the air and in the process it is converted from calcium hydroxide to calcium carbonate, so in the long term there is not much difference. (By the way, that is why formulations which call for calcium hydroxide lime, for example Bordeaux mixture, always stipulate that the lime should be fresh.) Nonetheless, garden lime, or (probably even better) dolomite, would be the better first choice for making soil more alkaline.
There is no equivalent material for making soil more acid. Sulphates in general, e.g., ferrous sulphate, magnesium sulphate, aluminum sulphate (hydrangea blueing agent) or ammonium sulphate will all have a fairly rapid acidifying effect but it is not particularly long term. Elemental sulphur lasts longer because it is slowly converted into sulphates by the actions of soil bacteria and water, but for the same reason it is significantly slower in its initial action. A good alternative, however, is to use compost. Compost is naturally acidic and, as stated earlier, improves the buffering of the soil both with regard to pH and also nutrients and water retention.
Conclusions
Gardening is a very rewarding pursuit and you don't need to be a chemist to be a good gardener. Nonetheless, sometimes just a little background knowledge can help to give greater insight and avoid problems that can otherwise lead to much frustration and lost plants. In this way it can make gardening an even more rewarding pastime and hobby.
Mike Hammer has been interested in both science and gardening since early childhood. The former interest was encouraged by his parents and the latter by the privilege of growing up on a 2 acre property which in the 1950s was semi-rural (although now well inside the suburbs). He studied electrical engineering at the University of Melbourne, graduating in 1975 with bachelor's and master's degrees. Since then he has worked as a research engineer and manager for Varian Australia, a high technology manufacturer and exporter of scientific instruments. Mike and his wife, Inge, always dreamed of living on a large property in the mountains but still close to the city. In 1989 a chance remark from a business colleague led them to look into a 6 acre property for sale at Sassafras on Mount Dandenong. It turned out to be the encapsulation of their dream - half temperate rain forest with a creek, and half rhododendron jungle (from plantings in the 1920s and 1930s). Residents there since 1991, they've been happily building a new house and redeveloping the extensive garden.