Does Scale Type and Density of Rhododendron Species of Section Vireya Have Any Relationship to Stomata?
Erik T. Nilsen
Department of Biological Sciences, Virginia Tech
Blacksburg, VA
David Webb
Botany Department, University of Hawai'i at Manoa
Honolulu, HI
Introduction
Organisms living in a terrestrial environment face a significant dilemma. Oxygen and carbon dioxide must be acquired without losing too much water or the organism will die from desiccation. This situation occurs because gases are absorbed by dissolving in water on a surface of the organism. The surface area of the organ where gases dissolve must be large so that an adequate amount of gas can be acquired to meet the needs of the organism. A large surface area is required because the concentration of important gases is relatively low in the atmosphere. For instance, the most important gas for plant growth (carbon dioxide) has an average concentration of only 0.0038 percent of the atmosphere. Terrestrial organisms enclose this large wet surface area for gas exchange in the interior of the body to minimize water loss. In insects, the gas absorbing structures are the tracheal tubes, which are connected to the atmosphere through holes on the insect's surface called spiracles. In mammals, lungs are the gas absorbing structures that are exposed to the atmosphere through the trachea, sinuses and mouth. Mesophyll cells in the interior of leaves serve as the gas absorbing surface for most terrestrial plants including
Rhododendron
species. Small apertures, called stomata, minimize water loss and allow gases to diffuse into the leaf interior. Clearly, plants have done a good job solving the dilemma of water loss during carbon gain because there are a wide variety of different leaf forms and many large and fast growing species.

|
Each stomata is composed of a hole called the stoma that is surrounded by two guard cells that control the size of the stoma. In general, the larger the stoma the larger the rate of carbon dioxide diffusion into the leaf. However, the larger the stoma the greater the amount of water vapor that can diffuse out of the leaf through the stoma. The balance of carbon gain to water loss through stomata (water use efficiency) is one of the most important dilemmas faced by terrestrial plants. Therefore, leaves have many independent checks and balances that regulate the size of the stoma. We will not be considering the physiological checks and balances on stoma in this article. Instead, we will focus on structural aspects of stomata on leaf surfaces in relationship to other leaf surface features.
Trichomes are cellular structures that extend outward from the epidermal surface of leaves. Two broad categories of trichomes can be identified based on their overall morphology. Leaf hairs are trichomes that extend more vertically than horizontally from the leaf surface often taking on the shape of a hair. These structures are commonly composed of individual, elongated epidermal cells, but at times they can have basal cells as part of their structure. Leaf hairs are found widely among
Rhododendron
species (particularly in subgenus
Hymenanthes
) on the abaxial side (underside) of leaves and are commonly called the leaf indumentum (Cowan 1950). Scales are trichomes that extend more horizontally than vertically and are often multicellular. The presence of leaf scales is an important character used in taxonomic treatments of the genus
Rhododendron
that separates the two largest subgenera. Plants in subgenus
Hymenanthes
(elepidotes) do not have leaf scales, but those plants in subgenus
Rhododendron
(lepidotes) have leaf scales. Further, variation in the morphology of leaf scales is used as an additional taxonomic character. For example, the key for subgenus
Vireya
is divided into 7 subsections partly based on the morphology of scales (Sleumer 1966).
1
Variation in the number of stomata per leaf area (stomatal density), average size of stomata, stomatal dispersion pattern on the leaf surface, presence of scales and trichomes, and climatic conditions can influence the movements of gases in and out of the leaf interior. In this article, we present data from more than 50 species (Table 1) out of the greater than 100 species growing in the ground at Mitch and Betsy Mitchell's garden in Volcano Village, Hawaii, USA. Growing all species in one common garden minimizes any environmentally induced difference in the structure of stomata, scales and leaves among species. Therefore, in a common garden, differences in leaf morphology and anatomy (phenotype) among species are predominantly due to genetics.
We started with the supposition that scales can influence the diffusion of gases though stomata. Therefore, if the structure, density, and dispersion of stomata are important for gas diffusion through stomata then there should be an influence of scale structure and density on the density, structure, and dispersion of stomata on the leaf surface. Based on this supposition we evaluated the following hypotheses. First, we expected that there would be a normal distribution of stomatal density classes among species in the common garden because we were sampling a large number of species from one monophyletic section of
Rhododendron
. Second, we predicted that stomatal density would be higher for species with small leaves. This is based on the theory that a set number of stomata are established early in development. As the leaf expands the cells of the epidermis between the stomata expand and this reduces the density of stomata per leaf area in larger leaves. Third, we predicted that the average length of stomata would decrease as the average density of stomata increased among species. Previous research has shown that there is often a negative relationship between the density and size of stomata. Fourth, we hypothesized that stomata would be randomly distributed for all scale types except stalked, large peltate (a circular scale with a short and stout stalk) and cushion scales (a peltate scale in which the circular cap is swollen into a cushion shape). This was based on our proposition that only tall, large-peltate and cushion scales would influence water movement from stomata. Therefore, dispersion of stomata should only be responsive to those three scale types. Fifth, we expected that stomatal pore index (a composite of density and size) would increase with an increase in leaf scale density for large scale types. The last hypothesis follows from the prediction that large scales slow down the movement of air across the leaf surface thereby increasing the humidity at the leaf surface. When leaf surface humidity is high, leaves can have a larger pore space for gaining carbon dioxide without loosing too much water.
| Table 1. An alphabetical list of species, accession, taxonomic placement, and authority for all species of Rhododendron subgenus Virey a used in this study. All taxa are growing at Mitch and Betsy Mitchell's garden in Volcano Village (elevation 1000m) Hawaii, USA. | |||
| Subgenus Rhododendron subgenus Vireya Species | Accession | section | Authority |
| armitii | RSF 87/073 | *Solenovireya | F.M. Bailey (1895) |
| apoanum | RBGE 19922792 | Malayovireya | Stein (1885) |
| aurigeranum | RSF 78/104 N287 | Euvireya | Sleumer (1960) |
| blackii | BOV 154 | Euvireya | Sleumer (1973) |
| brookeanum var. kinabaluense | RSF 318 | Euvireya | Argent, A.L. Lamb & Phillipps) Argent (1995) |
| bryophyllum | RSF | Phaeovireya | Sleumer (1960) |
| buxifolium | Richard Currie, NZ | *Malesia | Low ex. Hook. f. (1852) |
| carringtoniae | Bill Moyles, USA | Solenovireya | F.Muell. (1884) |
| celebicum | EWS 3-00 | Euvireya | (Blume) DC. (1839) |
| christi | RSF 79/031 | Euvireya | Foerste (1914) |
| commonae | Bill Moyles, USA | Malesia | Foerste (1914) |
| culminicola | Pukeiti | Euvireya | F.Muell. (1889) |
| fallacinum | Pukeiti | Malayovireya | Sleumer (1960) |
| gardenia | Neil Puddey, NZ | Phaeovireya | Schltr. (1918) |
| goodenoughii | RSF 83/063 | Solenovireya | Sleumer (1960) |
| himantodes | RBGE 1978 | Malayovireya | Sleumer (1940) |
| inconspicuum | Pukeiti | Malesia | J.J. Sm. (1915) |
| jasminiflorum | RSF 78/102 | Solenovireya | Hook. (1850) |
| javanicum | RSF 78/089 | Euvireya | (Blume) Benn. (1838) |
| javanicum -yellow | Neil Puddey, NZ | Euvireya | (Blume) Benn. (1838) |
| kochii | Bovees 10/00 | Euvireya | Stein (1885) |
| leptanthum | RSF 85/043 | Phaeovireya | F.Muell. (1889) |
| lochiae 'Devils Thumb' | Neil Puddey, NZ | Euvireya | F.Muell. (1887) |
| lochiae , pink | RSF | Euvireya | F.Muell. (1887) |
| loranthiflorum | RSF | Solenovireya | Sleumer (1935) |
| lowii | Frank Doleshy, USA | Euvireya | Hook.f. (1852) |
| macgregoriae | RSF 79/032 | Euvireya | F.Muell. (1891) |
| malayanum | RSF 97/077 | Malayovireya | Jack (1822) |
| multinervium | Chuck Barry, USA | Solenovireya | Sleumer (1960) |
| pauciflorum | RBGE 19750119 | Malesia | King & Gamble (1905) |
| perakense | Pukeiti 11/01 | Discovireya | King & Gamble (1905) |
| phaeochitum | RSF 87/044 | Phaeovireya | F.Muell. (1889) |
| polyanthemum | RSF 94/333 | Euvireya | Sleumer (1963) |
| radians | RSF 97/063 | Solenovireya | J.J.Sm (1920) |
| rarilepidotum | RBGE | Euvireya | J.J.Sm (1934) |
| robinsonii | RSF 83/066 | Euvireya | Ridl. (1909) |
| rugosum | RBGE 19801263 | Euvireya | Low ex Hook.f. (1852) |
| santapaui | BOV V309 | Pseudovireya | Sastry et al. (1968) |
| saxifragoides | RBGE 1993 0920 | *Sacifragoidea | J.J.Sm. (1915) |
| sessilifolium | RBGE 1974/1757 | Euvireya | J.J.Sm. (1934) |
| stapfianum | RBGE | Solenovireya | Hemsl. Ex Prain (1911) |
| stenophyllum | RBGE 1980/1190 | Euvireya | Hook.f. ex Stapf (1878) |
| sumatranum | RBGE 19881440 | Euvireya | Merr. (1933) |
| supurbum | RSF 78/094 | Phaeovireya | Sleumer (1960) |
| womersleyi | BOV | *Linnaeopsis | Sleumer (1960) |
| wrightianum | RSF-97 | Malesia | Koord. (1912) |
| x nervulosum = crassifolium x stenophyllum | Joan Bodenham, UK | Euvireya | Sleumer (1940) |
| x planecostatum = bagobonum x crassifolium | BOV | Euvireya | (Sleumer) Argent, A.L.Lamb and Phillips (1988) |
| x- sheilae = abiietifolium x buxifolium | RBGE | Euvireya | (Sleumer) Argent, A.L. Lamb & Phillips (1961) |
| yongii | RBGE | Euvireya | Argent (1982) |
| zoelleri 'Decimas' | Joan Bodenham, UK | Euvireya | Warb. (1892) |
| zollingeri | Pukeiti | Albovireya | J.J.Sm. (1910) |
| BOV=Bovees Nursery, Oregon, USA; EWS= E. White Smith; Pukeiti=Pukeiti Rhododendron Trust, New Plymouth, New Zealand; RBGE=Royal Botanical Garden Edinburgh, Scotland; RSF= Rhododendron Species Foundation, Federal Way, Washington, USA. | |||
| * Solenobireya, Euvireya, Malesia, Saxigragoidea, and Linnaeopsis are subsections of the section Euvireya. | |||
| Table 2: Mean stomata and scale characteristics of the abaxial (bottom) surface of leaves from Rhododendron species in subgenus Vireya growing at Mitch and Betsy Mitchell's garden in Volcano Village, Hawaii, USA. | ||||||
| Vireya Species | Stomata Dispersion* | Stomata Density | Stomata Length | Stomata Pore Index | Scale Type | Scale Density |
| armitii | 1 | 229.9 | 0.0207 | 0.0977 | 2 | 6.7 |
| apoanum | 4 | 269.5 | 0.0181 | 0.0856 | 4 | 27.2 |
| aurigeranum | 2 | 265.6 | 0.0219 | 0.1272 | 5 | 14.9 |
| blackii | 1 | 158.5 | 0.0215 | 0.0732 | 1 | 6.2 |
| brookeanum var. kinabaluense | 1 | 171.6 | 0.0201 | 0.0697 | 5 | 16.1 |
| bryophyllum | 1 | 192.8 | 0.0191 | 0.0700 | 7 | 10.3 |
| buxifolium | 1 | 158.5 | 0.0286 | 0.1302 | 5 | 12.4 |
| carringtoniae | 2 | 192.1 | 0.0205 | 0.0807 | 3 | 7.8 |
| celebicum | 1 | 153.0 | 0.0263 | 0.1060 | 3 | 5.0 |
| christi | 1 | 271.9 | 0.0198 | 0.1045 | 3 | 8.9 |
| commonae | 1 | 208.1 | 0.0238 | 0.1032 | 1 | 2.5 |
| culminicola | 1 | 209.3 | 0.0232 | 0.1124 | 3 | 6.6 |
| fallacinum | 4 | 152.6 | 0.0200 | 0.1052 | 4 | 47.0 |
| gardenia | 1 | 183.2 | 0.0210 | 0.0814 | 7 | 10.8 |
| goodenoughii | 1 | 279.3 | 0.0222 | 0.1386 | 3 | 6.8 |
| himantodes | 4 | 408.9 | 0.0298 | 0.3630 | 4 | 25.3 |
| inconspicuum | 1 | 251.2 | 0.0238 | 0.1424 | 1 | 13.7 |
| jasminiflorum | 1 | 170.9 | 0.0314 | 0.1664 | 3 | 4.2 |
| javanicum | 1 | 195.6 | 0.0234 | 0.1064 | 5 | 4.5 |
| javanicum -yellow | 2 | 346.5 | 0.0203 | 0.1421 | 5 | 19.0 |
| kochii | 2 | 326.0 | 0.0186 | 0.1156 | 3 | 5.5 |
| leptanthum | 2 | 328.7 | 0.0197 | 0.1275 | 5 | 6.0 |
| lochiae 'Devils Thumb' | 1 | 118.7 | 0.0259 | 0.0801 | 3 | 4.3 |
| lochiae , pink | 1 | 157.8 | 0.0263 | 0.1089 | 3 | 4.8 |
| loranthiflorum | 1 | 241.6 | 0.0248 | 0.1498 | 3 | 3.4 |
| lowii | 2 | 192.1 | 0.0245 | 0.1152 | 3 | 6.2 |
| macgregoriae | 1 | 321.0 | 0.0250 | 0.0780 | 1 | 6.1 |
| malayanum | 2 | 323.9 | 0.0161 | 0.0848 | 4 | 8.3 |
| multinervium | 2 | 249.8 | 0.0227 | 0.1282 | 3 | 7.8 |
| pauciflorum | 1 | 215.5 | 0.0212 | 0.0963 | 2 | 5.0 |
| perakense | 2 | 138.4 | 0.0315 | 0.1368 | 3 | 8.1 |
| phaeochitum | 2 | 350.0 | 0.0151 | 0.0799 | 7 | 16.8 |
| polyanthemum | 1 | 112.5 | 0.0276 | 0.0868 | 6 | 25.3 |
| radians | 1 | 160.6 | 0.0232 | 0.0874 | 3 | 2.9 |
| rarilepidotum | 2 | 319.8 | 0.0201 | 0.1283 | 3 | 5.1 |
| robinsonii | 1 | 133.1 | 0.0233 | 0.0721 | 3 | 3.5 |
| rugosum | 1 | 160.6 | 0.0262 | 0.1103 | 3 | 9.1 |
| santapaui | 1 | 179.8 | 0.0214 | 0.0818 | 2 | 4.7 |
| saxifragoides -abaxial | 1 | 241.9 | 0.0248 | 0.1481 | 2 | 5.5 |
| saxifragoides -adaxial | 1 | 133.0 | 0.0256 | 0.0857 | 2 | 1.3 |
| sessilifolium | 1 | 161.9 | 0.0224 | 0.0812 | 3 | 6.8 |
| stapfianum | 1 | 76.3 | 0.0427 | 0.1364 | 7 | 7.8 |
| stenophyllum | 1 | 374.9 | 0.0207 | 0.1631 | 1 | 0.4 |
| sumatranum | 2 | 188.7 | 0.0259 | 0.1268 | 3 | 10.8 |
| supurbum | 1 | 190.8 | 0.0200 | 0.0761 | 3 | 12.4 |
| womersleyi | 1 | 240.9 | 0.0222 | 0.1195 | 2 | 8.6 |
| wrightianum | 1 | 118.7 | 0.0298 | 0.1053 | 2 | 3.3 |
| x nervulosum | 1 | 205.2 | 0.0197 | 0.0792 | 3 | 3.6 |
| x planecostatum | 1 | 181.8 | 0.0255 | 0.1182 | 3 | 5.0 |
| x- sheilae | 1 | 151.7 | 0.0245 | 0.0898 | 3 | 5.7 |
| yongii | 1 | 146.2 | 0.0221 | 0.0713 | 2 | 10.0 |
| zoelleri 'Decimas' | 2 | 257.6 | 0.0219 | 0.1444 | 5 | 9.0 |
| zollingeri | 3 | 510.5 | 0.0167 | 0.1419 | 2 | 30.9 |
|
* Stomatal dispersion types are: 1 = random dispersion 0% under scales, 2 = random dispersion and 10% under scales; 3= 50% of stomata under scales, 4= 100% of stomata under scales.
Scale types are: 1 = small, peltate and sunken, 2 = small peltate, 3 = large peltate, 4 = cushion with flange, 5 = large cuneate, 6= stellate, 7= dendroid (stalked) |
||||||
Methods
Five newly mature leaves were collected from each plant (one plant per species in most cases) and sealed in iplock bags. The leaves were refrigerated until analysis. Each leaf was measured (length, width, area) and the scales and trichomes examined before any anatomical measurements. We utilized a stomata impression technique for determining stomatal density. The leaf surface (between two main lateral veins) was painted with clear nail polish (Clairol model 504). When the polish was dry and hardened a narrow piece of clear adhesive tape (Scotch brand package sealing tape) was placed over the nail polish, pressed down firmly, carefully lifted to remove the dried sheet of nail polish (the peel), placed (with the peel facing upward) on a microscope slide and covered with a cover slip. The hardened nail polish peel was examined through a light microscope to see the imprints of leaf surface structures including scales and stomata.
Images of peels were photographed (Nikon Cool Pix 900) at full optical magnification of the camera in all cases (Figure 6). We used a 2.5 x Zeiss plan ocular for images of stomatal distribution on the leaf surface and a 10 x Zeiss plan ocular to determine the density of stomata. Moreover, we used a 16 x Zeiss plan ocular for images of the relationship between leaf scales and stomata. Five locations on each epidermal peel were selected at random for images. Our randomization technique was to move the microscope stage a random distance while not looking through microscope. Obviously damaged areas were rejected and extra images were taken to insure that five representative areas could be sampled for each leaf. Once stationary, we would focus and take images.
We used an image analysis program, with a point counting capability (Image-J, NIH), when counting stomata to determine their density. An image of a stage micrometer (taken at the same magnification as the sample) was used to determine the total leaf area in each image. A similar technique was used to determine the density of scales. The mean size of stomata was determined by measuring the length of all stomata in the images recorded using the 10x ocular (usually between 15 and 35 stomata). The stomatal pore index (SPI) was calculated for each image as: D (L
2
). Where D = stomatal density, and L = mean stomatal length (Parlange and Waggoner 1970).
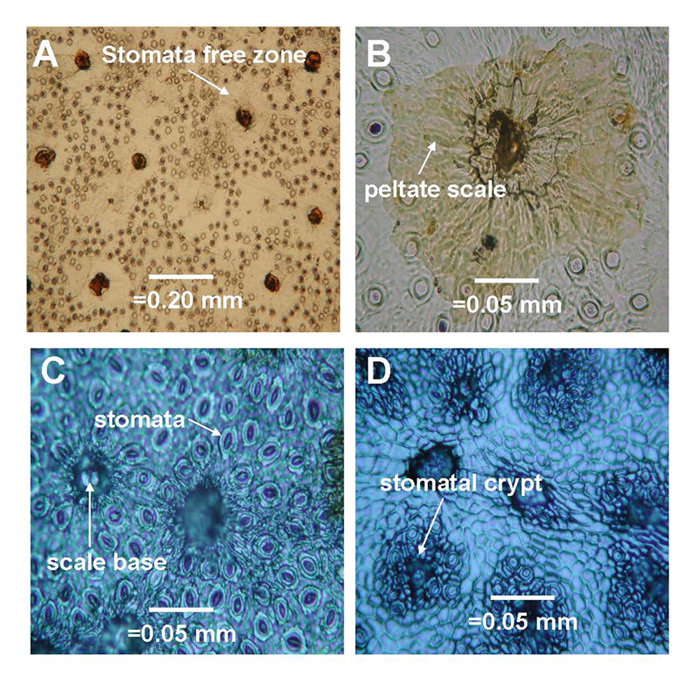
|
|
Figure 6. Representative images of stomata dispersion patterns on leaves of some species
which represent basic dispersion patterns for 50 species of Rhododendron subgenus Vireya growing at Mitch and Betsy Mitchell's garden in Volcano Village, Hawaii, USA. (1) Scale avoiding dispersion pattern of R. rarilepidotum ; (2) Partial scale avoiding dispersion pattern of R. kochii ; (3) Random dispersion pattern of R. zollingeri ; (4) Scale loving dispersion pattern of R. himantodes . Scale base = the basal cells remaining after the scale has been removed. Stomatal crypt = The indentation on the abaxial leaf surface that contains all the stomata and the base of each scale for species in section Malayovireya . |
Results and Discussion
Leaf morphometrics:
Leaf length varied from below 1.0 cm (e.g.,
R. womersleyi
) to more than 20.0 cm (i.e.,
R. sessilifolium
) and leaf width varied from approximately 0.5 cm (e.g.,
R. himantodes
) to over 7.5 cm (e.g.,
R. supurbum
). The ratio of leaf length to width remained fairly constant at approximately 2.5, reflective of a lanceolate leaf form, except for a collection of species that had a high length to width ratio (above 5.0) reflecting their linear leaf form (e.g.,
R. himantodes
,
R. stenophyllum
). It is important to note that the linear leaf form was found for species from different subsections of subgenus
Vireya
suggesting multiple independent evolutionary events that resulted in a linear leaf form.
Leaf area varied from about 0.5cm
2
to over 85cm
2
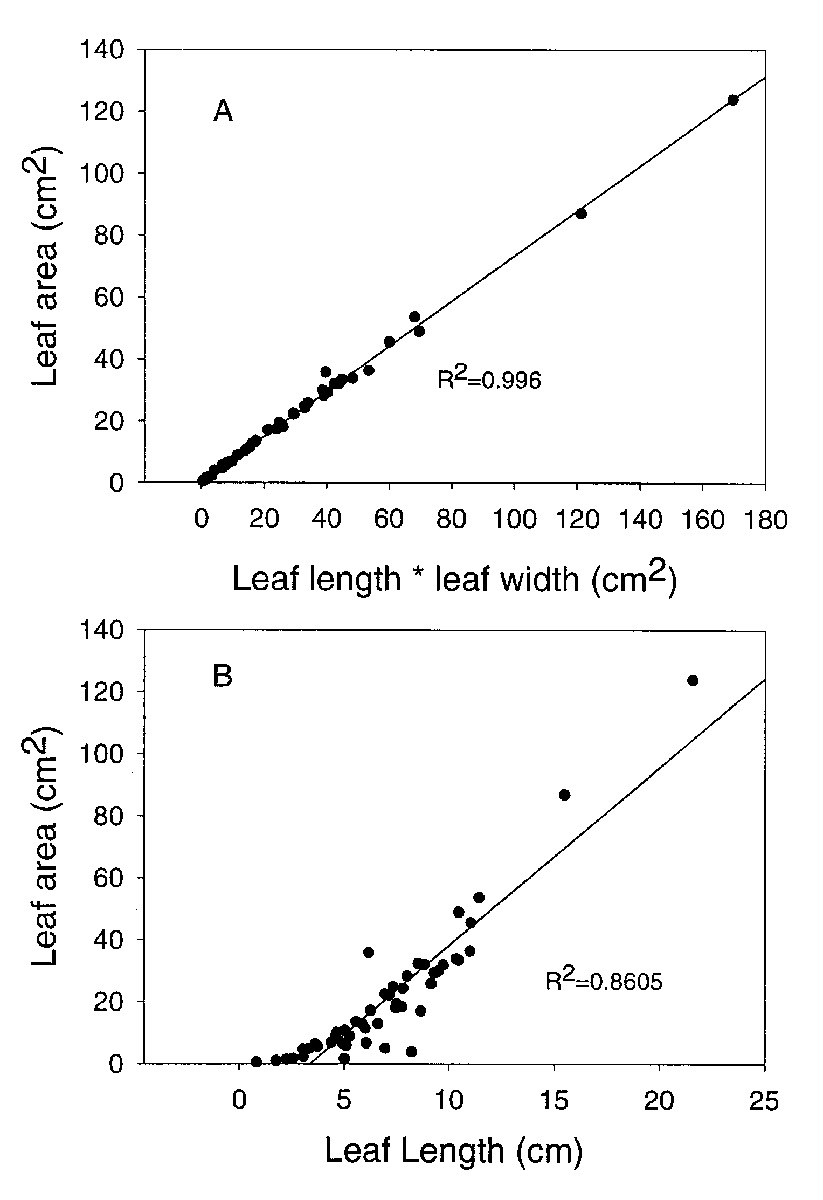
among species we studied. Leaf dimensions can be used to predict leaf area. The best correlation was found between leaf length times leaf width and leaf area (r
2
= 0.996; Figure 1A). However, a significant association was also found for the regression of leaf area on leaf length alone (r
2
= 0.861; Figure 1B). Therefore, measurements of leaf dimensions can be used as a surrogate for leaf area.
|
|
Figure 1: The relationships between leaf dimensions and leaf area for 50 species of
Rhododendron
subgenus Vireya growing at Mitch and Betsy Mitchell's garden in Volcano Village, Hawaii, USA. A. leaf length times leaf width vs. leaf area; B. leaf length vs. leaf area. |
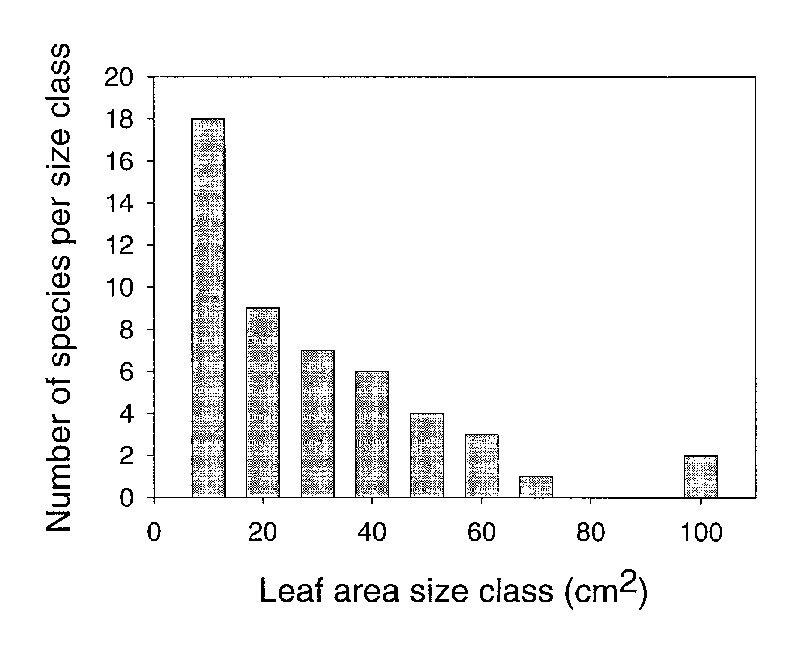
There was a skewed distribution of average leaf area among species (Figure 2) evaluated for this study (Kolmogorov-Smirnov normality test failed p=0.0003). The median leaf area was 18cm
2
. Only six of the species we studied had a leaf area greater than 50cm
2
. Our sample was selected to represent the variation in leaf size among species. Therefore, our data suggest that a majority of species in
Vireya
have relatively small leaves and there is a exponential decrease in the number of species with increasing leaf size.
Density and size of stomata among species:
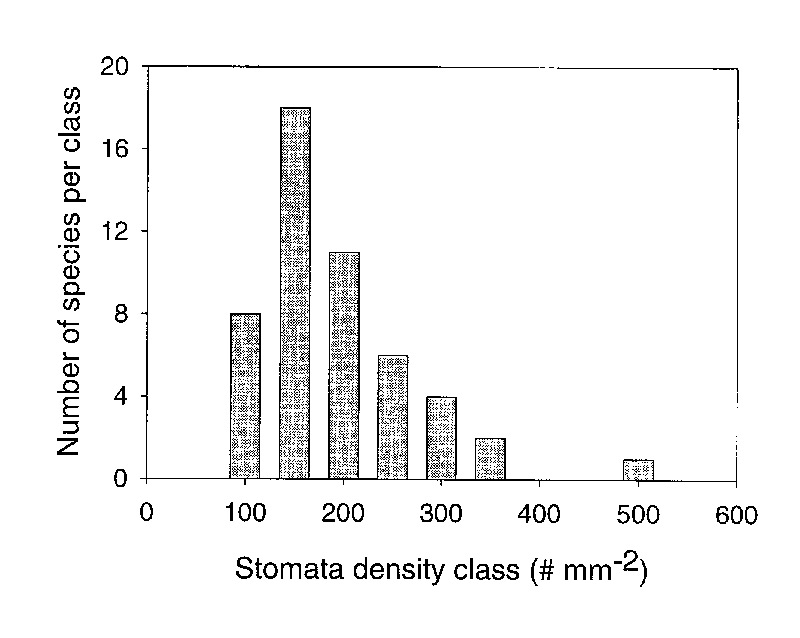
Stomatal density ranged from a low of 76.3 per mm
2
(
R. stapfianum
) to 510 per mm
2
(
R. zollingeri
) among all species we evaluated in this study (Table 2). The distribution of densities was not normal (Kolmogorov-Smirnov normality test failed p=0.006). The median density of stomata was 192.5 and the mean density was 219.3 11.9 mm
-2
which is an average stomatal density for leaves of broadleaf evergreen plants (Kelly and Beerling 1995). Therefore, our first hypothesis was rejected because the distribution of densities was not normal and most species had between 100 and 200 stomata per mm
2
(Figure 3).
|
|
Figure 2: A histogram of the number of species in different leaf area classes for 50 species
of Rhododendron subgenus Vireya growing at Mitch and Betsy Mitchell's garden in Volcano Village, Hawaii, USA. |
|
|
Figure 3: The number of species within stomatal density classes for 50 species of
Rhododendron
subgenus
Vireya
growing at Mitch and Betsy Mitchell's garden in Volcano Village, Hawaii, USA. Density classes are arbitrarily established as increments of 50 from 100 to 500 stomata per mm 2 leaf surface. |
All species were hypostomatous (stomata on the bottom surface only) except for
R. saxifragoides
, which was amphistomatous (stomata on both leaf surfaces). A predominance of hypostomatous leaves in
Rhododendron
species is expected because hypostomatous leaves are characteristic for plants with entire leaves and for those plants that commonly inhabit a subcanopy environment (Peat and Fitter 2002). The stomatal density on the adaxial (top) leaf surface of
R. saxifragoides
was 65% of that on the abaxial (bottom) leaf surface (Table 2). When the stomata on both leaf surfaces are summed for
R. saxifragoides
, the result (377 per mm
-2
) is higher than most of the other species tested (48 of 51 species had stomatal density below 350). The amphistomatous nature of
R. saxifragoides
is unique among all species of
Rhododendron
we have examined (over 200 species to date). Plants with amphistomatous leaves are characteristic in regions where carbon dioxide can become limiting to growth such as in high light habitats and for plants with small leaves (Peat and Fitter 2002). A more complete examination of
Rhododendron
species for high light alpine regions would be required to determine a broader view of this trait in the genus
Rhododendron
.
Climatic conditions during leaf formation can influence leaf morphology and stomatal density. If conditions are unfavorable (water stress, low nutrients, cold) stomatal density can increase because cells between stomata do not expand as much as they would under optimum conditions. However, the variation in stomatal density among species in this study is most affected by the genetics of the species because all species are grown in a common garden that has a seasonally constant climate. Small differences in stomatal density between species or among leaves of one species may be due to random, micro-environmental variation within a common garden. However, we can conclude from our data that species with particularly low stomatal density (100-150 per mm2) such as
R. polyanthemum
,
R wrightianum
and
R. stapfianum
have genetically determined lower stomatal density than those species with high (350-500 per mm
2
) observed stomatal density such as
R. phaeochitum
,
R. himantodes
, and
R. zollingeri
.
The length of stomata varied among species from 0.015mm to 0.043mm (Table 2). Stomatal size decreased with an increase in stomatal density (Figure 4). The negative relationship between stomatal density and stomatal size lends credibility to the data set because this is a well established relationship that is expected. As a consequence of this negative relationship, the stomatal pore index (SPI) remains fairly constant with a mean value of 0.109 0.028 (1 standard deviation) among species (Table 2).
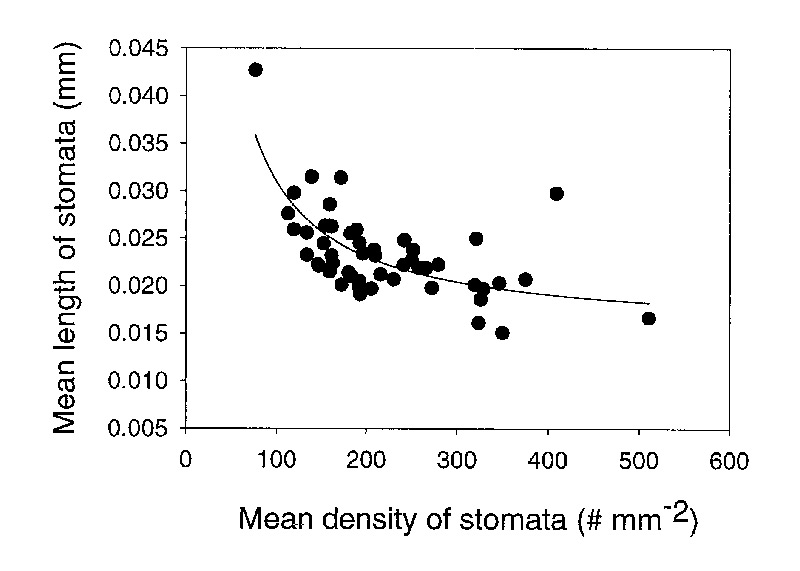
|
|
Figure 4. The relationship between the density and size (length) of stomata for 50 species of
Rhododendron subgenus Vireya growing at Mitch and Betsy Mitchell's garden in Volcano Village, Hawaii, USA. |
Stomatal density was not significantly associated with leaf area. For example, R. stapfianum and R. himantodes have a similar leaf area (6.09 and 3.89 cm 2 respectively) but a very different stomatal density (76.3 and 408.9 stomata mm -2 respectively). However, there was a constraint on the data. Larger leaves (above 40 cm 2 ) were constrained to lower stomatal density while smaller leaves had a wide diversity of stomatal densities (Figure 5). Consequently, our second hypothesis was partially supported because stomatal density of large leaves was constrained to smaller values but there was no significant association between leaf area and stomatal density. In contrast, our third hypothesis was accepted because stomatal size increased with a decrease in stomatal density. Combining our results from analyzing both hypothesis 2 and 3 suggests that the total amount of water lost by a leaf is proportional to the size of the leaf and total plant leaf area is a good predictor of potential water use by each species.
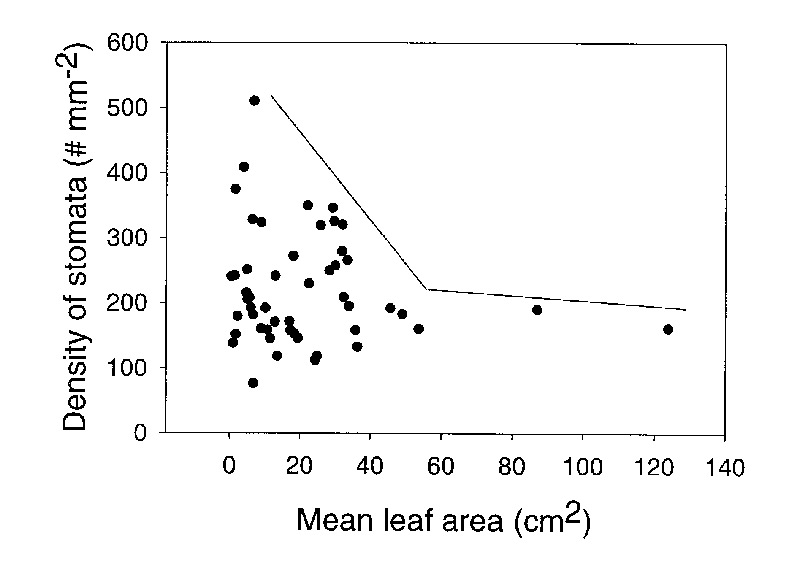
|
|
Figure 5. A plot of the relationship between mean stomatal density and mean leaf area for 50 species of
Rhododendron subgenus Vireya growing at Mitch and Betsy Mitchell's garden in Volcano Village, Hawaii, USA. The lines on the plot represent a constraint on the distribution of data. |
Patterns of stomata dispersion among species:
Four major types of spatial dispersion of stomata on the epidermal surface were observed among the species we studied (Table 2, Figure 6). Scale avoiding (Type 1) occurred when stomata were randomly dispersed on the leaf surface in all areas except in the immediate region of a scale. Usually, there was a distinct stomata free region around each scale. The extent of the stomata free area encircling scales varied among species. Partial scale avoiding (Type 2) occurred when most stomata were randomly distributed and about 10-20% of the stomata were found under the outer edge of peltate scales. In this case there was no stomata free zone encircling each scale. Random dispersion (Type 3) occurred when stomata were distributed across the leaf surface unaffected by epidermal scales. In this case about 50% of stomata were under the influence of scales. Scale loving (Type 4) occurred when all stomata were found under scales. Usually, the stomata of type 4 dispersion were located within a surface depression (crypt) under each scale.
Most species we studied exhibited type 1 or 2 stomatal dispersion, which represents a minimum potential influence of scales on stomatal function because of physical separation between the scale and the stomata. Scales on plants with stomatal dispersion 1 and 2 could only influence stomatal function indirectly by their influence on the air movement and humidity of the leaf boundary layer. Those species with type 3 or 4 stomatal dispersion were found only for species in subsection
Malaovireya
. The flange of cushion or peltate scales with type 4 dispersion could physically close over stomata stopping all gas exchange. However, scales could only impact 50% of the stomata in leaves with type 3 stomatal dispersion. Therefore, species in subsection
Malayovireya
are likely to be more drought resistant than species in other subsections because of the ability of scales to preclude water vapor loss from stomata.
Our fourth hypothesis was not supported because the most common type of stomatal dispersion (type 1) was not a random dispersion pattern. It was most common for stomata to be dispersed away from the immediate influence of scales. This result suggests that the major function of scales is not the regulation of water loss except in species from section
Malayovireya
.
Scale type and density:
Scales were classified into 7 types based on their potential influence on leaf surface humidity. This classification scheme is similar to that presented by others (e.g., Cowan 1950, Sleumer 1963, Argent 1988). We assumed that larger and taller scales would have a larger influence on leaf surface humidity than smaller shorter scales. This is because the larger taller scales would slow movement of air along the leaf surface. Our classification of scales was the following from least to most influence on leaf surface humidity: (1) small sunken peltate, (2) small peltate, (3) large peltate, (4) cushion, (5) cuneate, (6) stellate, (7) dendroid with a stalk. Large peltate and cuneate scales were most common (56%) among all species we studied (Table 2). Very few species had stellate (2 species) or dendroid (3 species) scales in the 50 species that we studied. One species (
R. stapfianum
) had hair shaped trichomes in addition to stalked, dendroid scales.
Relationship between stomatal density and scale density:
Scale density (Table 2) varied from 2.9 mm
-2
(
R. radians
) to 30.9 mm
-2
(
R. zollingeri
). Scale types 1-3 had lower densities than scale types 4-8 (Figure 7). The data were not normally distributed. Thus, significant differences in density among scale types were tested with a Kruskall-Wallis One Way Analysis of Variance on Ranks and a Dunn's method was used for pair wise comparisons. The difference in median values among scale types was statistically significant (p=0.0113). The scale density of types 1, 2, and 3 were significantly lower than scale types 4, 5, and 7. Therefore, the larger, taller scales were found at higher densities than the smaller and shorter scales.
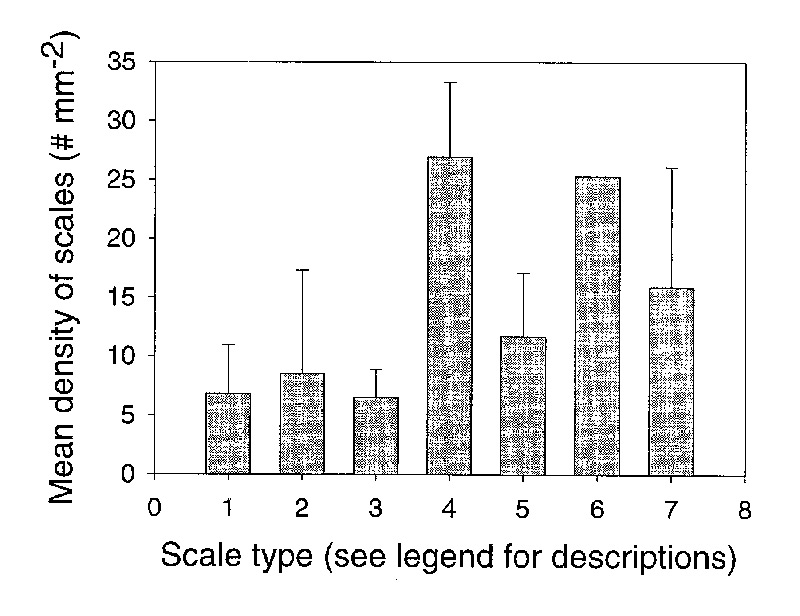
|
|
Figure 7. The relationship between scale type and scale density for 50 species of
Rhododendron
subgenus Vireya growing at Mitch and Betsy Mitchell's garden in Volcano Village, Hawaii, USA. Scale types are: (1) = small, peltate and sunken, (2) = small peltate, (3) = large peltate, (4) = cushion with flange, (5) = large cuneate, (6) = stellate, (7) = dendroid (stalked). Error lines represent 1 standard deviation for each scale type. No error line is represented for type 6 because only 1 species was found with this scale type. |
The density of stomata increased with an increase in the density of scales (Figure 8A). The strongest relationship was found for leaves with scale densities above 10 scales per mm2 (see Figure 8A). There was one outlier ( R. polyanthemum ) in this relationship. Stomata of R. polyanthemum are scale avoiding and the scales are stalked dendroid. These data suggest that the tall dendroid scales may be dense enough to influence the boundary layer of the leaf and reduce leaf water loss. There was no significant relationship between scale density and stomatal pore index (Figure 8B). This is because stomatal pore index is relatively constant (between 0.06 and 0.16) for most species. The one outlier was R. himantodes that had an exceptionally high stomatal pore index in relationship to scale density. This may be a result of the stomata in crypts covered by cushion scales. When leaves with large scale types (types 4-8) are analyzed separately from those with small scales (type 1-3) there is no change in the relationship between scale density and stomatal pore index. Therefore, our fifth hypothesis was rejected because stomatal pore index was relatively constant when scale density and type varied significantly.
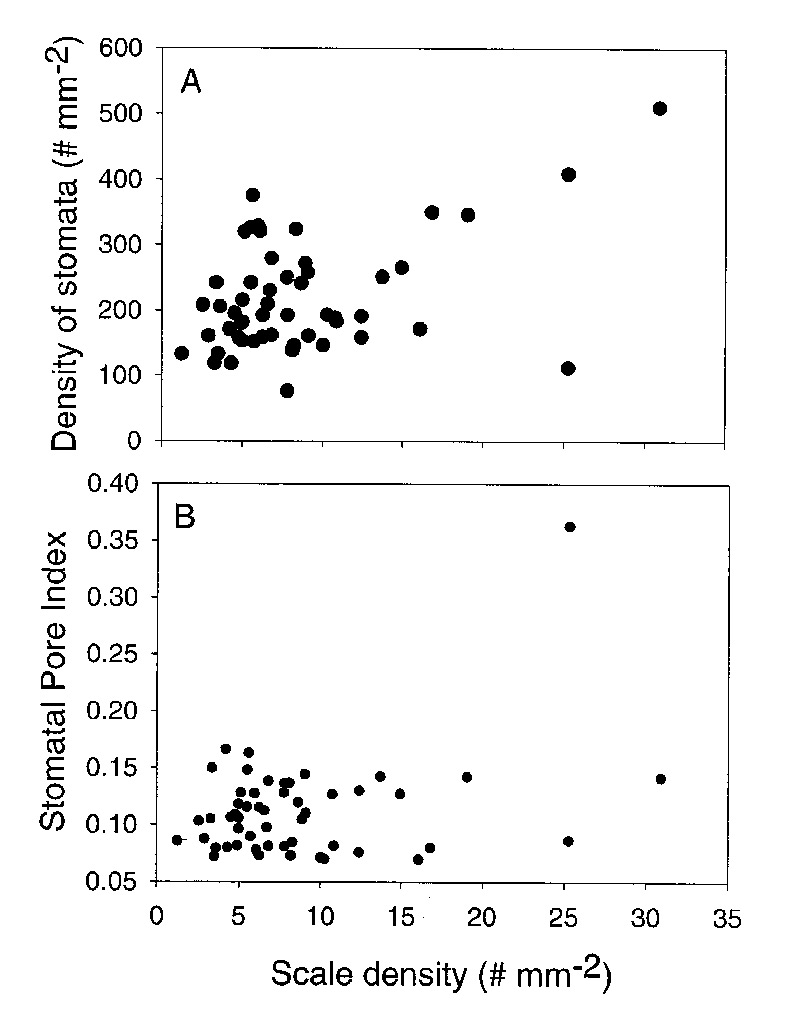
|
|
Figure 8. A comparison of scale density and stomatal density for 50 species of
Rhododendron
subgenus Vireya growing at Mitch and Betsy Mitchell's garden in Volcano Village, Hawaii, USA. A. The relationship between the densities of stomata and scales. B. The relationship between Stomatal Pore Index and scale density. |
Concluding Remarks
The significance of scales to leaf function has not been established by previous research. Some possible significances include reducing vapor demand by increasing the humidity in the air above stomata, reducing the palatability of leaf tissue to arthropod herbivores and increasing leaf reflectance of light from the adaxial surface (top of the leaf). We began this study with the supposition that scales on the abaxial leaf surface would influence the humidity of air close to the leaf surface causing a change in structural properties of stomata on leaf surfaces. Thus, we evaluated relationships between scales and stomata across a diversity of species from subgenus Vireya that had a wide range of scale types and densities. Predominantly, our evidence suggests that there is minimal effect of scales on structural aspects (dispersion, density, size) of stomata. In many species we studied stomata were distributed to minimize the influence of scales. Moreover, there is no significant relationship between stomatal pore index and scale density for all types of scales. However, exceptions to this thesis were found. Species of subsection
Malayovireya
(e.g.,
R. himantodes
) have scales that can have a strong effect on the function of stomata because the stomata are clustered in crypts below scales. This dispersion pattern occurs for species of
Rhododendron
subsection
Maddena
as well (personal data not presented). Further work on the relationships between stomatal function (transpiration) and scales is needed before a theory about scale influence of stomatal function could be finalized.
Vireyas
are fascinating species because of their horticultural possibilities and their ecological complexity. They inhabit a wide diversity of habitats in southeastern Asia, they have a diversity of plant architecture, and a diversity of leaf types. This is ideal for studying the relationships between leaf morphology and leaf function. We plan to continue our work on
Vireya
species to learn more about their basic biology and to address some important theories about the relationship between leaf form and function.
1 The taxonomic classification system used in this paper is that introduced by George Argent in his publication Rhododendrons of subgenus Vireya , Royal Horticultural Society, 2006.

|

|
|
|
Rhododendron laetum
Photo by David Webb and Erik T. Nilsen |
Rhododendron rarilepidotum
Photo by David Webb and Erik T. Nilsen |

|

|
|
|
Rhododendron goodenoughii
Photo by David Webb and Erik T. Nilsen |
Mitch Mitchell in his garden in Hawaii.
Photo by David Webb and Erik T. Nilsen |
Acknowledgements
This research was funded by a grant from the American Rhododendron Society Research Foundation, a grant from the Walker Fund administered by the Rhododendron Species Foundation, and by the G.P. Wilder fund for distinguished Botanists at the University of Hawaii. Our deepest gratitude goes to Betsy and Mitch Mitchell. They provided the plant resources, encouragement and friendly support. Without their help this project could not have been completed.
Literature cited
Argent, G.C. 1988.
Rhododendrons of Sabah
. Sabah Parks Publication #8, Kota Kinabalu, Sabah, Malaysia.
Argent, G.C. 2006.
Rhododendrons of subgenus vireya
. Royal Horticultural Society, London, 382pp.
Cowan J.M. 1950.
The Rhododendron Leaf. A study of epidermal appendages
. Oliver and Boyd, Edinburgh. 70pp
Kelly C.K. and D.J. Beerling. 1995. Plant life form, stomatal density and taxonomic relatedness: A reanalysis of Salisbury (1927).
Functional Ecology
9(3):422-431.
Parlange, J.Y. and P.E. Waggoner. 1970. Stomatal dimensions and resistance to diffusion.
Plant Physiology
46:337-342
Peat H.J. and A.H. Fitter. 2002. A comparative study of the distribution and density of stomata in the British Flora.
Biological Journal of the Linnaean Society
52:377-393.
Sleumer, H. O. 1966.
An Account of Rhododendron in Malaysia
. P. Noordoff Limited, Groningen, The Netherlands.