Why Is it Pink, Daddy?
Paul Rogers
Aloha, Oregon
Traditional methods of animal and plant breeding have accomplished much, albeit haphazardly. But in the past 150 years or so we've learned how it really works in general, and in some detail. We can apply modern biological knowledge to improve our rhododendron hybridizing success.
One can hybridize by dabbing pollen on a pistil. That's awfully "hit and miss." Directed hybridizing means identifying:
1. Achievable goals ("Can we get a true blue, or orange, rhody?"),
2. Means to the ends (“What's missing in what we have & how do we get it?"),
3. Useful raw materials ("What crosses would provide needed genes?"),
4. Results which illuminate the pathway ("What do our seedlings tell us about getting what we want?").
All four are based on recognition. In the natural world, in all fields, certain observations are also revelations. They reveal important information about the processes involved. The key is insightful recognition. All four will be found in the following discussion.
I am convinced Mother Nature has displayed many of her secrets to hybridizers over the years - we didn't notice. We didn't understand how Mother Nature does her tricks. Learning to recognize what she does will make us better observers. That takes some work and study on our part to meet her halfway. This is about practical application of the science, about recognizing when Mother Nature reveals something important, and knowing how to make use of it.
How do we get from colorless water, carbon dioxide, sunlight, and a few minerals to red, yellow, purple, and blue pigments? This article will be about the essential elements of the details we know, but much simplified. Although not restricted to color and pigmentation, that will be the general focus. However, we start here because the process from genes to observable effects works the same way in all cases, whether pigmentation or plant habit.
The stuff we should know is based in biology, genetics and biochemistry in particular. Our results are rooted in those sciences. But what we need to know isn't that complicated! Really. It can be simplified to relevant facts. People who don't understand what DNA is can understand what a "parts list" is. The essential function of DNA is simply a "parts list." That's it. How hard is that? We don't need to know chemistry or genetics, but we need
to understand what they do
. Understanding the fundamentals makes us better able to recognize and apply what we observe.
Money goes where money is. So perhaps it's not too surprising that hasn't included much research into rhododendron flower pigmentation and genetics. Yet there has been research into high value plants and plant products, e.g., roses, petunias and wine, which can be of use to us. Pigments are even involved in cancer research.
All flowering plants have been found to make their pigments the same basic ways. While we don't have as many details as we'd like about rhodies, they aren't entirely unique and we do have details from more commercially significant plants. Right now, let's be clear that pigmentation and intensity are different things. Pinks and reds, lavenders and purples differ in intensity, but may and probably do have exactly the same pigment. Intensity, blotches, spots, and picotee may have to wait for another day.
What Pigments?
There are two main classes of plant pigments, carotenoids and flavonoids. Aurones seem to be quite rare, of doubtful relevance in rhodies. The table below page classifies the characteristics of the main and subsidiary pigment classes.
| Class | Chemical nature: | Examples: | Colors: |
| Carotenoids | Oil soluble terpenes. | Found in: Leaves, Roots (carrot), Fruits (tomato), Flowers (rarely except Compositae) | Red, Orange, Yellow |
| Carotenes | Oil soluble aromatic isoprenes. | Alpha carpteme, Beta carotene, Vitamin A, Lycopene | Yellow, Orange, Red |
| Xanthophylis | Oil soluble hydroxylated terpenes | Capsaicin, Lutein, Saffron | Yellow |
| Aurones | Water soluble furans. | Rare but found in: Snapdragons, Dahlias | Yellow |
| Flavonoids | Water soluble phenolds | Found in: Flowers, new growth | Visible UV |
| Anthoxanthins | Water soluble, neutral pigments & copigments | Chalcones, Flavones, Flavonones, Flavonols | Colorless, Yellow |
| Anthocyanins | Water soluble, acidic, i.e., pH sensitive, ionic pigments. | Delphinidin, Pelargonidin, Peonidin, Petunidin, Malvidin | Red, Purple, Blue |
Carotenoids:
Carotenoids in rhodies are probably only seen when leaves turn yellow before falling off, or the few variegated rhodies. They are not common in flowers, except of the
Compositae
, e.g., dandelions, sunflowers. But we need a test. Is yellow in rhodies from carotenoids, anthoxanthins, or even possibly aurones? I haven't found that answer in the literature. How can we tell? It would be a simple "science fair" experiment. Collect a lot of yellow petals from different species/hybrids, and macerate each in a blender with some water. Using a Pyrex glass saucepan and a double boiler, add the petals, water, and mineral oil. "Cook" slowly. Oil soluble carotenoid pigments will transfer to the oil, as when cooking chili or spaghetti sauce. If the oil layer stays clear, then they're flavonoids.
Flavonoids:
Flavonoids are heavily researched for the florist trade and because they have antioxidant and anti-cancer properties. For example, catechin is a product of flavonoid synthesis and one of the anti-oxidants in green tea.
Anthoxanthins:
One of our first questions must be the role of anthoxanthins in pigmentation. Anthoxanthins are generally colorless to light yellow. This is a reasonable explanation for
R. lutescens
,
R. keiskei
,
R. hanceanum
, and elepidote yellows. What about
R. luteum
, Mollis azaleas, or Exbury golds and oranges? Why are they so intense? We don't know yet. There are some hints in the literature and some possible examples in the rhododendron family that golden yellows might be due to a higher pH. More about some possibly relevant observations below, where the pH sensitive anthocyanins give us some clues.
Anthocyanins:
Six anthocyanins (cyanidin, pelargonidin, delphinidin, peonidin, malvidin, and petunidin), and their derivatives make flowers' red, purple, and blue pigments and some of the reddish pigments in leaves. Don't get hung-up on the names of the anthocyanins. The name usually refers to the genus from which the pigment was first extracted and chemically identified. Malvidin was identified in mallows, but is in cyclamen and grapes (
Vitis vinifera
), and colors red wine. Cyanidin pigments blue bachelor buttons, but also red roses, boysenberries, and Himalayan blue poppies (
Mecanopsis betonicifolia
).
Some species of flowering plants can synthesize more than one pigment, some can't. Petunias can synthesize petunidin and delphinidin. Crocus can synthesize petunidin and malvidin.
Vaccinum pedifolium
synthesizes peonidin, petunidin, and malvidin. Roses can make cyanidin and peonidin, and some make pelargonidin, e.g., 'Tropicana'. Roses and rhodies aren't known to make delphinidin. (Sun-Tory & Florigene used gene manipulation to get delphinidin into roses. Blue roses are coming!) Visual appearance of mixed pigments can be deceiving. Again, a simple "science fair" level experiment can separate mixed pigments. Paper and/or liquid-phase chromatography use small differences in solubility to separate pigments. It's not particularly difficult. It will tell us whether two different hues are different pigments, or the same pigment at different pHs. Anybody got a desperate high school student looking for a science fair exhibit?
Anthocyanin pigments aren't particularly intense or stable on their own, but they can make more stable and intense complexes with colorless flavonoid "co-pigments," and sometimes metallic ions, e.g., aluminum in
Hydrangea
. We are still learning about the co-pigments. (Color intensity is also a function of the articulars of the cell vacuoles where pigment resides.)
The pH Effect
However, we needn't look for different pigments to explain the different hues of rhody flowers. Species with anthocyanin pigments can have different hues because they have different levels of acidity in their petal tissue, but the same pigment. Bluebells are blue, right? Except when they're pink! After the core pigment, pH is the next most important factor influencing hue.
How are rhodies like hydrangeas? The hue of rhody flowers is just as dependent on pH as hydrangea flowers. Some plants don't control petal tissue pH, e.g., hydrangea. Whatever pH is at the roots gets transported to the flower. When we "sweeten" the soil, we aren't changing the pigment molecule. Pink and blue hydrangeas have the same pigment. However, most plants control the pH within the flower petals, i.e., pigment hue is consistent in different soils. Work with petunias suggests they have seven genes controlling the pH in petals. Some plants are intermediate. (Read Greer's description of 'Aloha'.)
Depending principally on pH, cyanidin runs from red through purple to blue. Pelargonidin varies from orange to brick red. Delphinidin varies from purple to blue. Malvidins are generally purple. Petunidin is red, and peonidin is purple, but don't seem to change greatly. The general rule of anthocyanins is the lower the pH in the petal tissues, the redder the pigment, and the higher the pH, the bluer, all with one pigment molecule. Peonidin is desirable to rose breeders because, unlike cyanidin, it doesn't "blue" with age, i.e., higher pH.
Bad news: we don't know exactly which pigments rhodies can produce. Are reds cyanidin or peonidin? Are purples, cyanidin or malvidin? Can we find evidence? When we see a range of pigmentation in related rhodies going from bright red, through darker reds, magentas, and purples to bluish, we should suspect a pH variation of a common cyanidin pigment molecule. In the
Arborea
subsection: arboreum is red,
lanigerum
is magenta, and
niveum
is purple, which suggests cyanidin from low to neutral pH. In the
Pontica
subsection:
maximum
is pink,
smirnowii
is magenta, and
ponticum
is purple. In
R. hippophaeoides
, var.
occidentale
(aka
fimbriatum
) is red-purple; the
hippophaeoides
Sunningdale clone is blue.
If we apply this to hybrids, reddish purple 'Anah Krushke' would have lower pH, blue-purple 'Smokey', higher pH. 'Mrs Furnival' and her daughter with the pink petals and crimson blotch should have lower pH than 'Mrs G.W. Leak' with the slightly more lavender petals and burgundy blotch. In R. augustinii hybrids, pinkish 'Bergii' indicates low pH; 'Blaney's Blue' indicates high pH. 'Cynthia' shows higher pH than 'Pink Pearl'. This seems to be evidence that rhodies' principal pigment is cyanidin.
Seeing and Using pH in the Garden
Does it matter whether we call it pH or pigment, since we want an end result? Yes. Depending on what we're trying to produce, if we recognize the clues we're seeing that inform us about pH, we might introduce genes to change the pH. There are many somewhat purplish reds, e.g., 'Cynthia', 'Nova Zemblya', which could be made better reds by a cross on something with lower pH tissues. Knowing that hue is the combination of pigment and pH means we can go looking for a parent that will influence pH even if it doesn't have the pigment we want. We may find the pH we need in something with an entirely different color! We can separate the pH and pigment with proper breeding.
Here's an example of a cross that exposed genes for pH. Cross pink and crimson 'Mrs Furnival' with pale yellow
R. wardii
and what do you get? Nearly white, very pale lavender, 'Peeping Tom' with a dark purple blotch. Why is 'Peeping Tom' interesting? Where did the purple come from? We can certainly hypothesize a reasonable answer based on knowledge of pigment and pH. It must have come from the red pigment (cyanidin) of 'Mrs Furnival'.
R. wardii
must have brought more alkalinity to the petal tissue. The (low pH) red blotch became (neutral pH) purple. The rest of the petal also shifted from pink to lavender but seems to have lost some intensity. Again this red to purple shift suggests cyanidin. Oh, to see the F2 of 'Peeping Tom' selfed! Bulgin doesn't list any.
If we can see these things in the garden, what might we do with them? The
scyphocalyx
hybrid 'Clarke's Golden Gate' has quite a golden throat and purplish lips. It's reasonable to hypothesize the purplish anthocyanin pigment (cyanidin) indicates the petal tissue has a high pH, and this would suggest some yellow pigments in rhodies are golden at higher pH. One could work on separating out the purple anthocyanin genes, but leaving the high pH genes in F2, to get a golden yellow elepidote.
Ah, but there's a fly in the ointment. If yellow rhodies come from anthoxanthins, which in the primary systhesis pathway (see below) are neutral molecules, why should they be sensitive to pH, and higher pH produce more golden tones? Good question, but first we need some confirmation that higher pH, evidence of which we see in purple and bluish elepidotes, can lead to the production of the golden yellow we see in the Exbury azaleas. How do the Exbury's do that? We need it in elepidotes!
There's hope for "blue breeders." Cornflower blue is a complex of three cyanidin molecules, three flavones, one ferric ion, one magnesium ion, and two calcium ions at a high pH. If we can get cornflower blue in a rhody that will be good enough! In order to explain the pinky augustinii like 'Bergii', again most likely the blue-purple in augustinii is cyanidin, so the base for cornflower blue is already there. It's simplest to hypothesize all colored augustinii have the same cyanidin pigment genes, and the differences in hue are due to different genes that change the pH of the petal tissues. So we're not breeding for pigment, but for pH. Is the pH of bluish augustinii as high as can be? Could that produce a bluer flower?
We can find out. We should try to find a cross that shows high pH petal tissues, i.e., a purpley one from a family of reds, or a golden one from a family of yellows. Don't worry about crossing yellow on blue, I'll show later why this isn't like mixing paint. Remember 'Peeping Tom'.
In the
Cinnibarina
subsection, consider orange Blandfordiiflorum Group, and purple Purpurellum Group. Both appear to be higher pH versions of their yellow
R. cinnabarinum
ssp.
xanthocodon
and red Roylei Group kin respectively. Looking to change the pH of a lepidote? By the way, here we see the independent effects of genes for anthocyanin pigment and pH. Mendel discovered that a century and a half ago.
Blotches sometimes seem to have their own independent set of pigments. But in most cases pH seems to affect all petal tissue, both petals and blotches. Sometimes petals can have anthocyanins, the blotch anthoxanthins. Look on pg. 98 of Greer at 'Valley Sunrise' and 'Torlonianum', purple picotee with an orangey blotch, evidence high pH makes orange. Yellow blotches or spots in reds, such as 'Oregon Queen' on pg. 117, 'Llenroc' on 212, 'George's Delight' on 211, 'Wild Affair' on 223 show low pH.
It could be difficult trying to put a (low pH) red blotch on a (high pH) purple picoteed petal, or vice-versa. Finding that would be significant - the equivalent of picotee or blotching derived from pH rather than pigment. If you find one, it's important. Propagate it!
Producing Pigment
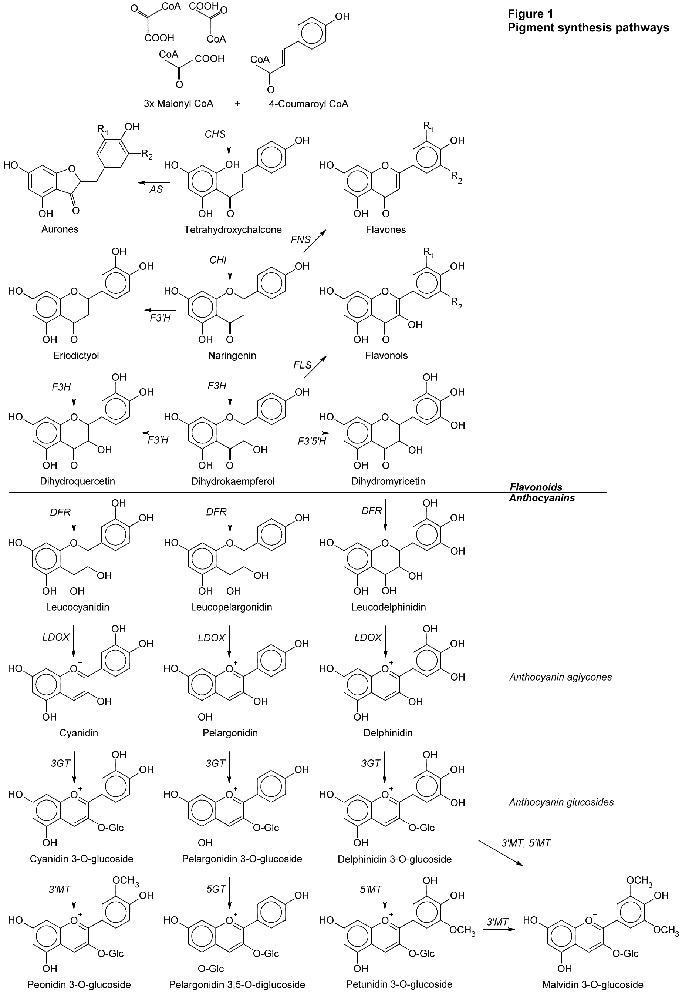
Pigments don't just spring up complete. They must be built, step by step. The path used by flowering plants is shown in Figure 1 below. In the figure, the arrows show the way of the synthesis. The italicized letters next to the arrows are the acronyms of the enzymes that perform each step.
This is the basic pathway used by flowering plants in general. In the anthocyanin pigments, the principal characteristics are based on the central backbone of six molecules shown in Figure 1 (cyanidin, peonidin, pelargonidin, delphinidin, petunidin, and malvidin). After this common part of the synthesis other substitutions are usually made in different species. I show just one example, the pelargonidin diglucoside.
|
Of the molecules shown in Figure 1, many are colorless, e.g., those with the "leuco-" prefix. In the flavonoids, some of the chalcones, flavonols, and flavones are yellowish. The path to red, blue, and purple anthocyanins passes through yellow pigments or their precursors. The anthocyanin aglycones are weakly colored and unstable. But notice the little "+" sign above the oxygen atom. This is the point at which anthocyanins get their pH sensitivity. As glucosides, the color gets more intense and more stable.
What are we to see in Figure 1? After the first step shown (CHS is a busy enzyme), each enzyme uses a precursor molecule, makes an incremental change, creating a new molecule. Different hues and shades of color on different species of plants depends on exactly which genes and enzymes they possess, and how the synthesis process makes its way down these paths with the enzymes it has.
We should see that peonidin is derived from cyanidin. Roses have been found to produce both. Petunidin and malvidin derive from delphinidin. Unless a species can go down the delphinidin path, it's not going to be making petunidin or malvidin either. We've already learned crocus can. Rhodies aren't known to. Delphinidin blue will have to come from a new "random" mutation.
A closer look at how this works answers many puzzles observed in rhody hybridizing.
It starts with a "gene." Genetics works the same in all but truly archaic forms of life (
Archaebacteria
) and organelles. Large catalogs of genes exist for many organisms. We wish there were for rhodies.
There is commonly a lot of confusion about what genes do. Forget the DNA, double helix stuff. That's what it is, and we're interested in what it does. It's the results that really interest us. A gene is effectively a "parts list" for the production of a specific protein. These proteins generally are, or are parts of, enzymes. Enzymes are an organism's biochemical synthesizers. They're the paths to pigment. Enzymes take an existing molecule and chemically transform it into a different molecule. Many steps are required to get from original "raw materials" to complex molecules. Think of a "bucket brigade." The enzymes are our firemen, and the buckets are our molecules.
Is the introduction of enzymes as the active agents a distinction without a difference? No, but the details are a complexity we can avoid. A gene's effects are limited to where it is found, within the nucleus of the cell. Enzymes are produced outside the nucleus, can be secreted through the cell wall, and can affect other cells.
Let me introduce the concept of "wild type." It is the genome of the prototypical example of a species, e.g., a "tabby" cat. It would help if we could establish wild types among mutually fertile varieties of rhodies. A recognized wild type gives everyone the same basis of comparison. For our purposes in hybridizing, where we can generally cross freely within the subgenera, lepidotes, elepidotes, azaleas, etc., we would want "prototypical wild types" that would encompass all the crosses we could make. If a species is defined by intra-fertility, we have entirely too many "species"!
A mutated gene creates alleles: the original wild type and a new "mutated" alternative. Both alleles are representatives of the same gene. Over time, there can even be multiple alleles. In virtually all cases, members of a true species have all the same genes, not necessarily all the same alleles. But of the important ones, yes; that's what makes them identifiably part of the species. Roses and rhodies almost certainly have a "gene for delphinidin," but the allele that's there produces an enzyme,
F3'5'H
, that doesn't work. Get used to using "allele" where most people refer to a "gene."
While we're speaking of, say, "a gene for cyanidin," which one is it? To get cyanidin in a red rhody,
CHS
,
CHI
,
F3'H
,
F3H
,
DFR
,
LDOX
, and
3GT
must all be working. Block any one of them and the cyanidin disappears. So the correct answer is "all of them." Conversely, which is "the gene for white"? At least potentially, "all of them." If the cyanidin disappears, there's no indication which enzyme isn't working.
We tend to overlook the entire complement of genes, and for purposes of discussion just refer to the mutant genes. Nevertheless, although people talk about a "delphinidin gene" or a "white gene," it can be misleading. I tend to avoid it. A plant with a delphinidin gene (are we talking about
F3'5'H
?) can simultaneously have a functioning cyanidin gene (
F3H
?). Several different genes can produce malfunctioning enzymes that break pigment synthesis, producing white. There isn't one "white gene," there are several. Don't forget the others!
Things would be pretty boring if only the wild type of all living things existed. Over generations, mutations, or "sports" as we call them in the plant world, happen within the genomes of individuals, getting passed down to descendants. A mutation is a change in the "parts list" of what makes up the proteins and enzymes of the individual. Sometimes the change affects the enzyme function, producing an observable effect, sometimes not.
We only observe the effect of the ones that do.
That is a surprisingly subtle point. Ignorance of this apparently obvious fact leads to much confusion.
Two things can happen. In some cases it has been shown that any substitution at a critical point within the enzyme can render it non-functional. It breaks the synthesis path. Referring back to Figure 1, it would seem that far back in their ancestry, roses and rhodies inherited a version of the
F3'5'H
enzyme that doesn't work, doesn't make dihydromyricetin and, subsequently, delphinidin. Since then, no mutation back to the original gene has returned to their genomes, making an enzyme that works. We can find places in Figure 1 where dysfunctional
LDOX
enzymes could block the formation of colored anthocyanins, making unpigmented flowers.
DFR
could block all anthocyanins, leaving the chalcones and flavonoids, and only the possibility of yellow pigments.
CHI
or
CHS
could block the chalcones and flavonoids and subsequently anthocyanidins, producing true white flowers without any pigment.
The other thing that can happen is the new enzyme works, mostly, but in a slightly different way. Again referring to Figure 1, examining the pathways involving dihydroquercetin and dihydrokaempferol, it seems a plant that can synthesize cyanidin should also be able to synthesize pelargonidin. Whether or not that happens depends on the
DFR
enzyme. Mutation has created some versions of
DFR
that are specific about which precursor molecule they can use. If it must be dihydroquercetin then pelargonidin will not be produced, for example.
I know this seems pretty complex, but this is why it is important. It explains why some of our crosses can produce such unexpected results. Suppose we crossed, for example, a white with a yellow. The white's
F3H
enzyme works, but it has a malfunctioning
LDOX
enzyme, accumulating colorless leucocyanidins. The yellow has a malfunctioning
F3H
enzyme, but its
LDOX
enzyme would presumably work if it ever got the colorless leucocyanidin. These parents are complementary. The seedlings get a functional
LDOX
from one parent, and a functional
F3H
from the other. They can synthesize a cyanidin and be red or purple. This is why hybridizing rhodies isn't like mixing paint. Realize this: just because one enzyme is "broken" doesn't mean the rest of them are!
A similar thing could happen with the crosses of two whites, referring back to the Bowers letter in the ARS Journal several issues ago. "Common observations of everybody's results seem to indicate that the 'wild' or lilac color of
R. catawbiense
, present in varying amount in almost all of the hardy hybrids, is very hard to get rid of. I have found that even the white clones (most of which are pinkish in bud), such as 'Catawbiense Album', 'Album Elegans' and 'Boule de Neige', when inter-crossed give a preponderance of purplish or lilac colored seedlings in the F1" (Bowers, JARS, 60:1:31, Winter 2006).
By the same token, if we were breeding yellows, crossing on white to "clarify things" wouldn't necessarily be helpful. Making pigments isn't like mixing paint. The fundamental process for making yellows is the same as the process for making whites. To produce both yellows and whites we must interrupt the synthesis pathway. Exactly where the whites' pathway is blocked isn't obvious. What genes the white may have beyond the blockage aren't apparent. Uninterrupted synthesis proceeds to reds and purples, and true blues if we had delphinidin. To stay yellow we need that
F3H
path to be blocked. In a white we can't tell if it is. We also don't get any clues about pH, as we might from a colored rhody.
Many species have yellow pigmented members, where the synthesis process doesn't make it all the way through. Quoting Greer:
R. souliei
: "creamy yellow, pink or rose, occasionally to almost white or soft, deeper rose".
R. lepidotum
: "pink to crimson or purple, greenish yellow, yellow or white." Do you see it? Those descriptions each reveal two mutations!
Bowers' examples of two whites producing colored seedlings, tell us we're dealing with two different broken enzymes, two different "genes for white." If we crossed two whites and got all white seedlings, then we learn both parents have the same blocked path, the same gene, and possibly the alleles. This is how geneticists built up their catalogs of genes. But even if that's not our goal, the information could be useful. It should be noted.
Mendel crossed purple flowered peas on white flowered peas in the first scientific experiments in plant hybridizing about 150 years ago. All the seedlings had purple flowers, so he called purple "dominant" and white “recessive,” terms still used today. Now we understand that the white had a malfunctioning enzyme in that list, and the purple just provided a working one to the seedlings. It's more useful to recognize the function of enzymes. Dominant and recessive aren't especially useful terms anymore, but to the extent they are used, they are compared to "wild type."
I've tried to keep this focused and on target, while showing what the science means to us in practical terms. Of the original question, I got to the red but haven't gotten to intensity (which admittedly is less well understood), or other important pigmentation effects, such as picotee. I'll leave those for another day. I've got more on seeing the effects of this science in the garden, and applying the science to hybridizing.
References
Bulgin, Lansing W. 1986.
Rhododendron Hybrids, a compendium by parent.
Craig, Donald. 2006. Nothing new under the sun.
JARS
60:1:30-34.
Greer, Harold. 1996. G
reer's Guidebook To Available Rhododendrons, species & hybrids
, third edition. Eugene: Offshoot Publications.
Paul Rogers, BS Chemistry, 1967, California State College - Long Beach, has been seriously raising animals, which must be bred and cannot be "vegetatively propagated," since he was 8 years old, using genetics since a high school student. He made the "mistake" of trying to identify some rhodies at a new home, and came under the influence of Tualatin Valley Chapter members. His main "project" is the production of a fragrant bright yellow.