JARS v63n2 - Collection of Winter-hardy Rhododendrons at the Central Siberian Botanical Garden, Russia
Collection of Winter-hardy Rhododendrons
at the Central Siberian Botanical Garden, Russia
Elena Chernykh and Tatyana Novikova
Central Siberian Botanical Garden
Russian Academy of Sciences
Novosibirsk, Russia
The objective of this project was to create a collection of rhododendrons that would be sufficiently
winter-hardy to grow well under the environmental conditions of Novosibirsk in Central Siberia. Here
in Siberia where weather conditions are extreme, we can not grow the diverse rhododendron cultivars
that grow well under milder climatic conditions. Therefore, to increase the diversity of rhododendrons
growing in our gardens, we need to take advantage of the intraspecific variability that exists within
species native to Siberia. Our first goal was to search for natural populations of Rhododendron
ledebourii Pojark, growing in the Altai Mountains to find clones with needed features. In its
natural habitat, this species grows both in the shade of forests and on open, stony slopes. It is
both winter-hardy and drought resistant, and tolerates heat and sun. While there is debate among
Russian botanists as to the names of the rhododendron species found in the Altai Mountains
(Alexandrova, 1975; Koropachinskiy and Vstovskaya, 2002; Malyshev, 1997), here we use the
classification accepted by this journal (Chamberlain et al., 1996).
The main reason we decided to focus on R. ledebourii from the Altai Mountains was that this
region is much closer to Novosibirsk than are the Lake Baikal and Russian Far East regions where
other interesting rhododendron species grow. The Altai is a mountain range in Central Asia where
Russia, China, Mongolia and Kazakhstan meet, and where the great rivers Irtysh, Ob and Yenisei begin.
The northwest end of the range is at 52° N between 84° and 90° E, and it extends southeast from there
to about 45° N 99° E, where it gradually becomes lower and merges into the high plateau of the Gobi
Desert. We worked in the northern part of the Altai Mountains, where both cold and dry conditions
occur and where cold resistance and drought tolerance would be expected.
During a spring field trip, we planned to document variation in flower size, shape and color and to
collect vegetative material for initiating in-vitro culture. From the literature, we learned, that
flower size could range from 2.5-4.0 cm in diameter, and intensity of color from pale to intense pink;
very rarely, plants with white flowers could be found. Two expeditions to the Altai Mountains were
organized:
1) In September 2003, we collected 11 samples of R. ledebourii seeds from different habitats
and sent them to the ARS. Also, 14 herbarium specimens were collected and sent to the Royal Botanic
Garden, Edinburgh, and
2) In May 2005, we documented an unexpectedly large variation in flower size, shape and color.
In addition to what we have done ourselves, other colleagues at our request collected rhododendron
seeds from other regions of Russia: near Lake Baikal (R. fragrans (Adams) Maxim. and R.
aureum Georgii); in the vicinity of Ulan-Ude, Buryatia (R. dauricum L.) and in the
southern part of the Russian Far East (R. sichotense Pojark, R. mucronulatum Turcz.
and R. schlippenbachii Maxim.).
All these seed samples were also sent to the ARS. In August 2006, one additional sample of R.
ledebourii was collected by colleagues from the Altai Mountains and was sent to the ARS.
1. Seed collecting trip to the Altai Mountains, September 2003
Our first field trip to the Altai Mountains in September 2003 had the goal of collecting seed
samples from the different ecotypes of R. ledebourii. We focused on three different habitat
types:
1) Stony slopes along the Katun River, including northeast facing slopes covered with birch and
southwest facing steppes;
2) Dry pine forest along the Katun River; and
3) A moist mixed forest along the Syema River.
On the stony slopes along the Katun River, we collected seven samples. Plants varied in the
persistence and autumn coloration (Fig. 1) of their leaves, in plant form, and in having fall
blooms.
 |
 |
|
| Figure 1: Stony slopes along the
Katun River. Site open to the sun but protected from the wind by rocks, altitude 500 m, beautiful compact R. ledebourii, 1 - 1.5 m tall, with red leaves. Photo by Elena Chernykh and Tatyana Novikova |
Figure 2: Rhododendrons with
attractive fall colouration growing in the moist mixed forest along the rocky banks of the Syema River; plants 2 - 5 m tall, alt. 400 m. Photo by Elena Chernykh and Tatyana Novikova |
R. ledebourii has an extensive range, occupying very different habitats. While collecting
seeds, we found populations adapted to quite different ecological conditions: shade, humidity,
drought, and sun. We observed healthy looking plants growing in humid mixed forests on acid soils
(Fig. 2), in dry pine forests, and even on dry grassy slopes on calcareous rocks in stony-cobble
soils (Fig. 4). In July 2006, rhododendron plants were photographed in the central part of Altai
Mountains, south of our 2003 trip, growing under extremely dry conditions (300 mm (about 12")
annual precipitation) on southwest facing slopes on rocks without any visible soil, fully
exposed to sun and heat (Figs. 3, 5).
For these reasons, we expect R. ledebourii to be a wonderful source for drought, heat,
sun and cold tolerance in a breeding program. However, to achieve specific breeding goals, it is
still best to select seed parents from certain habitats with known environmental characteristics.
McAleese and Rankin's (2000) study of wild rhododendrons growing on limestone in China indicated
that some rhododendron species are well adapted to high concentrations of available calcium in the
soil and can tolerate pH up to 8.6. Similar research is warranted with R. ledebourii, as
we expect certain populations of this species to be lime tolerant because of adaptations to
geological conditions where they occur (Baksht and Romanenko, 1971). Another species native to
Siberia, R. fragrans, also grows on limestone-derived soils and outcrops (Malyshev, 1997).
 |
| Figure 3 Dry southwest-facing slope,
2 m tall Rhododendron with dark-red leaves growing on top of a rock, altitude 700 m. Photo by Elena Chernykh and Tatyana Novikova |
 |
 |
|
| Figure 4: Central Altai: R. ledebourii
plants are dwarfed, (30 - 50 cm tall), have pronounced leathery glossy leaves, look healthy, and are able to produce new growth and seeds on calcerous rocks, alt. 1000 m. Photo by Elena Chernykh and Tatyana Novikova |
Figure 5: Central Altai: On this
south-facing slope, R. ledebourii grows from cracks in the rock; there is no visible soil. An example of extreme drought, heat and sun tolerance. Photo by Elena Chernykh and Tatyana Novikova |
2. Collections from other regions of Russia
Wild Rhododendron seeds from other regions of Russia were collected for us by colleagues from
other botanical gardens. Since they were organizing field trips for their own purposes (and were
supported from other sources), we only used our ARS grant specifically for the following
collections from other regions of Russia, which were sent to the ARS in 2003: R. dauricum,
collected in the vicinity of Ulan-Ude, Buryatia; and R. mucronulatum, R. sichotense,
and R. schlippenbachii from the Far East.
In March 2004, 14 herbarium specimens were collected from ten locations and a CD with pictures of
both collected plant material and the live plants from which the specimens were collected were
sent to the Royal Botanic Garden, Edinburgh.
In addition, seeds from the following Rhododendron species from the Lake Baikal region were sent
to the ARS in October 2004:
1) R. fragrans (Fig. 6): an evergreen small shrub with divaricate branches. Seed
samples of R. fragrans were collected in the Eastern Sayan Mountains, about 100
km west of Slyudyanka, the most western point of Lake Baikal, in the vicinity of Arshan,
where it grows on calcareous rocks only on open southern slopes in the subalpine
zone. In winter, these slopes usually get up to 80 cm of snow (ATLAS of Irkutsk
Region, 1962), but because it is often blown off by wind, these plants are very cold resistant.
Due to many glandular trichomes (hairs tipped with small glands containing oils), many parts
of the plants, but especially leaves and flowers, have a nice spicy fragrance. Leaves are
used by local people for making a tea with tonic properties.
2) R. aureum (Fig. 7): native to the southwestern part of the Lake Baikal region.
Seeds were collected in the Kchamar-Daban Mountains near the village of Tanchoy. These
plants grow on north-facing slopes in moss in the partial shade of Pinus pumila. Soils
there are well aerated and not compacted. This area has relatively high precipitation both
in winter and summer (600 - 800 mm year-1), and in the winter, slopes are covered
with 80 - 100 cm of snow (ATLAS of Irkutsk Region, 1962). In the summer, the air humidity
is high due to its close proximity to Lake Baikal. The plants are about 1 m high and are also
very cold resistant.
 |
 |
|
| Figure 6: R. fragrans. Photo by Evgeny Zibzeev |
Figure 7: Rhododendron
aureum Georgi. Photo by Elena Chernykh and Tatyana Novikova |
3. Field trip to the Altai in May, 2005
Our second expedition to the Altai Mountains took place in May, 2005, and focused on documenting
variability in flower size, shape and color and on collecting vegetative material from the most
attractive plants for initiating in-vitro culture. We visited the same sites where seeds were
collected in September 2003 and an additional site, close to Seminskiy Pass, where we actually
found the most variation in flower shape, size and color in a relatively small plant population.
We already knew, that there was considerable habitat variation among R. ledebourii
populations. In May 2005, we were excited to find quite a range of plants with distinctive
ornamental characteristics (Figs 8-14). Plants differed in many ways (Table 1).
| Table 1. Ornamental characteristics of R. ledebourii flowers. | |
| Characteristics | Variation |
| flower size flower shape flower color flares darker veining arrangement of stamens in the flower stamen length stamen color anther color petal lobes lobe edges spots or stripes on upper lobe, lobes length of pedicels number of flowers per flower bud number of flowers in a cluster |
from 2-5 cm across broadly campanulate to openly funnel shaped to flat-faced pale lavender to dark lavender, pale crimson to crimson to dark crimson in buds present or absent present or absent scattered bundle to tongue-shaped bundle shorter to longer than the length of petals white to pale rose to pink yellow turning white, pink turning blue absolutely smooth to wavy smooth or wavy present or absent 0.5-2 cm 1 or 2 maximum of 8 |
 |
 |
|
| Figure 8: Plant 1.5 m tall with
pale lavender single flowers, 3.5 cm across, with lavender filaments and blue anthers in a scattered bundle. The main features here are the smooth petal lobes and open funnel-shaped corolla that becomes flat with age. Photo by Evgeny Zibzeev |
Figure 9: Inflorescence
of mainly single flowers, 4.5-5 cm in diameter, with a nice pink color, filaments pink, scattered, with yellow anthers. Beautiful in appearance thanks to a broadly-funnel to saucer-shaped corolla that resembles apple flowers. Both the corolla shape and slightly wavy lobes with flares create an impression of airiness. Photo by Elena Chernykh and Tatyana Novikova |
 |
 |
|
| Figure 10: Crimson, broadly-campanulate to
openly funnel-shaped flowers with large corollas to 5 cm across, upper lobes with dark crimson spots, white filaments with yellow anthers, dark crimson pistils. Impressive clusters composed of two to four flowers. Photo by Elena Chernykh and Tatyana Novikova |
Figure 11: The most
floriferous plant we found. Campanulate to broadly-funnel shaped flowers with smooth lavender lobes, rather short, scattered lavender filaments with yellow anthers and short pedicels. Photo by Elena Chernykh and Tatyana Novikova |
 |
 |
|
| Figure 12: Small flowers,
2.5 - 3 cm across, deep lavender, campanulate to broadly-funnel shaped with scattered lavender filaments, yellow anthers, and dark purple pistils. Single flowers on relatively long pedicels loosely covering the plant were all in the same phase of flowering. Photo by Elena Chernykh and Tatyana Novikova |
Figure 13: Medium-sized
flowers, 3 - 3.5 cm across, pink flowers with smooth, emarginate lobes, flares; terminal clusters composed of four flowers. Photo by Elena Chernykh and Tatyana Novikova |
 |
||
| Figure 14: Large single flowers,
4.5 - 5 cm across, pale lavender, broadly funnel shaped to almost flat-faced; "inverse umbrella" resulting from a symmetrical broadly-open corolla with stamens arranged almost perpendicular to the corolla plane. Photo by Elena Chernykh and Tatyana Novikova |
4. Micropropagation Technique
During the field trip, branches with flowers and those with shoots were removed from selected
plants, sprayed with water, and covered with polyethylene bags. They were kept in cool boxes
for transport. To avoid etiolation of young shoots, they also received a couple of hours of
day light during the return trip while kept in bags. At the lab, the branches were kept at
room temperature in glasses with water under fluorescent lights to support the growth of
young shoots. They were loosely covered with polyethylene bags with small holes.
Young shoots were cut from the branches, rinsed for 15 min in running tap water and then
sterilized for 20 min in 5% solution of commercial bleach, containing 5% sodium hypochlorite.
This low concentration of sterilizing agent was chosen after multiple experiments with
different concentrations (from 2.5% to 10%) and exposure times (10 - 40 min), conducted
with plants growing at the Botanical Garden. Tissues of R. ledebourii proved to
be extremely sensitive to sterilizing agents, so the whole procedure is a compromise
with very narrow margins between achieving sterile undamaged explants and sterile dead
ones. After sterilization, shoots were washed in five changes of a 0.5% solution of ascorbic
acid, and then were kept in the same solution of ascorbic acid for 20 minutes. The
concentrated solution of ascorbic acid was filter-sterilized prior to being diluted with
sterile water. Tissues of R. ledebourii contain many secondary metabolites, so the
use of antioxidants is necessary to prevent post-sterilization browning. We found out
that it was more effective to wash explants in a solution of ascorbic acid to immediately
neutralize the sodium hypochlorite rather than to wash them first in water and then
transfer them to the antioxidant. This happened to be especially true with young
shoots.
After the shoots were removed from the antioxidant solution, leaves and apices were cut away
from the shoots, since we already knew that growing apices were always damaged by this process.
The cuttings were then either dropped into half-strength liquid Anderson (Anderson, 1984)
medium, containing 0.5 mg l-1 IAA, 2.5 mg l-1 2iP and 5 mg l-1 GA , or put on agar plates
of the same medium. To our great disappointment, we were unable to induce in-vitro growth
from any of the clones we carried to the lab. We could not overcome contamination of the
ex-plants, and so had to rethink our propagation strategy.
 |
||
| Figure 15: Pedicel buds uncovered
under the flower bud scales. Photo by Elena Chernykh and Tatyana Novikova |
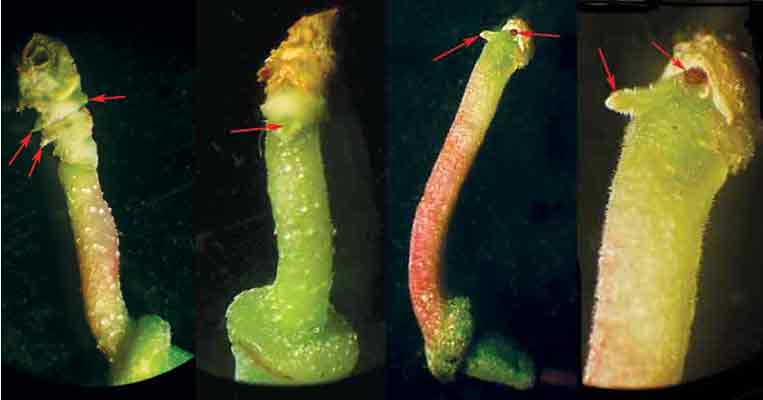 |
 |
|
| Figure 16: Arrows point to pedicel buds.
The image furthest to the right is a close-up of the image just to its left. Photo by Elena Chernykh and Tatyana Novikova |
Figure 17: Stem-like
structure regenerated from pedicel base. Photo by Elena Chernykh and Tatyana Novikova |
Pedicel Explant Experiments in February - June 2006
The following experiments were done in February 2006, with plants of R. ledebourii of
unknown origin grown in the Botanical Garden's greenhouse. The idea was to choose explants
other than shoots, that could be expected to be less contaminated if collected in the field,
that would be easier to transport from the field trip to the lab, and that were not as
sensitive to the sterilizing agent as are shoots.
We did not expect to learn that there are vegetative buds just at the base of some pedicels (Fig.
15).
Buds are situated either on the pedicel itself, at the point of its juncture with the transition
zone between pedicel and stem, or within this transition zone, in the axils of flower bud scales
(Figs. 15, 16). Some flowers have pedicel buds that can be seen with naked eye, some do not.
Even when the pedicel buds are absent, the base of the pedicel has meristem tissue that regenerates
under in-vitro conditions, reacting in three-five days to growth regulators in the culture medium
and producing stem-like structures (Fig. 17).
The ability of tissues at the base of the pedicel to regenerate by reacting positively to growth
regulators under in-vitro conditions was restricted to a rather short time period. Several days
after flower opening was found to be too late, since the pedicel buds either did not enlarge, or if
they did, they soon turned brown and died. The best time to cut flowers for this purpose was just
before they open to no later then two-three days after they open. Interestingly, as the pedicel
buds enlarge, the ovary also enlarges to three-four times of its original size. When the pedicel
buds stop enlarging, the ovary stops enlarging, too. The pedicel should not be cut off from the
ovary, as it then turns brown very fast.
To sterilize the pedicels, pieces of stems (one-two cm long) with attached flowers were cut off the
plant, wrapped in wet paper towels and brought to the laboratory. Petals, stamens, flower bud
scales and old leaves were removed, and pieces of stems with pedicels and ovaries were rinsed for
30 min in running tap water. They were then sterilized for 20 minutes in a 2.5% solution of
commercial bleach (containing 5% sodium hypochlorite), the minimal working concentration for
explants taken from the greenhouse; for those from outdoors, we used 5% bleach. Next, the plant
material was washed in five changes of a 0.5% solution of ascorbic acid and then immersed in the
same ascorbic acid concentration for 20 minutes. Afterwards, the pedicel was carefully cut off
from the stem just at the point of juncture in order not to damage its base, which bears the
regenerative meristems and any buds formed in the axils of the flower bud scales (Figs. 15, 16).
The stigma was removed, since it is sticky, has an uneven surface to help hold pollen grains on it,
and usually carries many fungal spores that the sterilizing agent may fail to reach.
The trimmed explants were then placed into half-strength Anderson liquid medium, supplemented with
0.5 mg l-1 IAA, 4mg l-1 2iP and 5 mg l-1 GA . Typically within four days, a "brush" of growing buds
can be observed at the base of the pedicel, even for those pedicels that did not have visible buds
before being placed into the growth medium (Fig. 17).
We found it was more convenient to collect flowers and flower buds in the field and to transport
them to the lab than to collect entire shoots with or without new growth, since they occupy much
less space, can be easily packed into a cooler, do not transpire as much water as young shoots do
and there is less risk of damage. There is one more key advantage: pedicels and pedicle buds stay
covered by the flower bud scales and as a result are not so exposed to contaminants as shoots are,
and so sterility can be more easily achieved.
After performing experiments with the material taken from the greenhouse in February 2006, we
eagerly awaited the arrival of spring to be able to take explants from plants growing outside in
the Dendrarium of the Botanical Garden. However, when the time came for these rhododendron
plants to bloom, we experienced a terrible disappointment: no flower buds were opening. They were
mostly all killed by the extremely cold winter of 2005 - 2006: -30 C low temperatures began in
November, when there was still no snow; in January we had -40 C for two weeks in a row, followed
by several more periods of -30 C throughout the rest of the winter. The only ones that survived
were some single flowers on the lowest branches that had overwintered under the snow. However,
though these flower buds survived winter frosts, they were subsequently affected by a drought in
the spring, since after the snow melted in mid April, there was no rain until the end of May, only
sunny weather with dry winds. Actually, the flowers seemed normal, but while collecting them for
our experiments, we noticed unusual insect behavior: a bee would touch a flower and then immediately
fly away, would try another flower and immediately again leave. We suspected there was little or no
nectar in the flowers, since nectar formation can be strongly impacted by drought.
Nevertheless, these collected flowers went through the preparation and sterilization procedures
described above. We managed to sterilize ovaries with pedicel buds and those without pedicel buds,
and after one week in culture they showed no signs of contamination, but ultimately they were not
viable. They reacted by creating some small enlargements after being treated with growth regulators
but shortly thereafter turned brown. Pedicels without buds did not show any meristem growth activity.
The explants behaved like those that were taken much too late a stage, though we collected them at
an early stage, just before the flower buds should have opened.
In summary, based on our preliminary results, we suspect that ovaries with pedicels are more reliable
and easier to handle as explants for in vitro culture of R. ledebourii from the wild than
are young shoots. However, further experiments need to be done to create a working protocol for
collecting material and for efficient in-vitro propagation that can withstand different environmental
challenges. Special experiments should be done under controlled conditions to determine whether
pollination and fertilization of the flower before it has been cut off would influence the ability
of pedicel and pedicel buds to be good explants.
References
Alexandrova, M.S. 1975. Rhododendrons in the Flora of the Soviet Union. Moscow, Nauka
(In Russian).
ATLAS of Irkutsk Region. 1962. Moscow - Irkutsk. Head Department of Geodesy and Cartography
(In Russian).
Anderson, W.C. 1984. A revised tissue culture medium for shoot multiplication of Rhododendron.
J. Amer. Soc. Hort. Sci. 109: 343-347.
Baksht, F.B., and M.F. Romanenko. 1971. Geological Essay. The Mountain Altai. Tomsk State
University: 63-72. (In Russian)
Chamberlain, D., R. Hyam, G. Argent, G. Fairweaather and K.S. Walter. 1996. The Genus Rhododendron,
its classification and synonymy. Royal Botanic Garden, Edinburgh: 181 pp.
Malyshev, L.A.1997. Rhododendron L. Flora of Siberia. V.11: 16-19. (In Russian).
Koropachinskiy, I., and T. Vstovskaya, 2002. Woody Plants of Asiatic Part of Russia.
Novosibirsk, Geo. (In Russian).
McAleese, A.J., and D.W.H. Rankin. 2000. Growing rhododendrons on limestone soils: Is it really
possible? J. Amer. Rhododendron Soc. 54: 126-134.
Acknowledgements
The project was supported by the Research Foundation of the American Rhododendron Society (ARS).