Studies of Factors Inhibiting the Rooting of Rhododendron Cuttings
Bernard Thomas Bridgers
Research conducted under the auspices of the
Agricultural Experiment Station, University of Maryland
Part One
Introduction
The rhododendron is one of our most outstanding broad-leaved evergreens. It comes to us from the arctic and tropics in a splendid assemblage of pygmies of a few inches high to medium-sized trees of sixty feet, with a color range which challenges even the roses.
Through the efforts of the plant breeders the color range is being further expanded, fragrance has been added to the flowers, and the size of the flower clusters has been increased in diameter. These improvements, coupled with hardiness of the plant and comparative freedom from insects and diseases, have made a remarkable increase in demand.
With the true American species, however, the color range falls into white and pale magenta, and the brilliant colors are found only in the hybrids. To the average home owner the hybrids are not available due to the fact that cost of production is high which is reflected in the price of the plants. Since hybrid rhododendrons do not come true from seed, the only methods of propagation left to the nurseryman are grafting, layering, and leaf-bud cuttings.
Grafting is an expensive operation in this country because it involves considerable labor and specialized equipment. In Europe where labor is cheap this method of propagation can he used, but when these plants are imported into the United States the costs of shipment and losses suffered during transit make the price per plant approximately the same as those grown in this country.
Layering is a method ideal for the home propagator, where a few plants are desired. However, for the commercial nurseryman simple and air layering are expensive and slow processes, and only a relatively few plants may be obtained from a layered mother plant at any one time.
Leaf-bud cuttings have been used as a method of propagation, and the nurseryman experiences little difficulty in rooting; however, the subsequent breaking of the dormant buds is unpredictable, and for this reason the method is not widely used.
The only way the price of a rhododendron plant will he lowered is by reducing the labor involved in production, and the only way this can be attained is by propagating by stem cuttings. Attempts have been made to determine the factors inhibiting the rooting of rhododendron cuttings. As yet the absolute causal factor of inhibition has been undetermined.
For this reason further investigations have been made to determine the cause by chemical, environmental, anatomical, and physiological studies.
Review Of Literature
The propagation of hybrid rhododendrons has been considered from both commercial and scientific standards for more than a century. Results of these investigations offer at the present time much controversy and disagreement as to the best procedures to follow in layerage, graftage, or cuttage.
Layerage is a slow but satisfactory method of propagation, and as suggested by Hanger (14), it is probably the best method for the amateur propagator. Cleft grafting is the main commercial method in the United States, but there is considerable diversity as to desirable species of understock. In a search for compatibility between stock and scion, Kley (12) recommended that
Rhododendron caucasicum
and
R. smirnowii
be used instead of
R. ponticum
. Chadwick and Gunesch (5) propagated Cunningham's White to be used for understock. They felt that since this plant was found to be tolerant of alkaline conditions, its use as understock would extend the range of rhododendron culture. To prevent suckers of grafted plants Hanger (14) used
R. ponticum
in root grafting, a method also considered important as every chance was provided for the scion to eventually initiate roots.
Propagation by cuttings using conventional methods has been unsatisfactory; several extraordinary techniques have been tried with various results. Small (29) treated cuttings of undetermined species with periodic watering using hot and cold water and acetic acid of different concentrations but did not obtain rooting. Applications of potassium permanganate and acetic acid to the rooting media gave unfavorable results as described by Chadwick and Gunesch (5). When cuttings of mature wood were treated by solution immersion with 0.05 per cent potassium permanganate for 15 hours, there was an increase in per cent rooting. Succulent and semi-mature wood showed no such response to this treatment.
Zimmerman and Hitchcock (36) treated cuttings of Catawbiense hybrids with a series of root inducing chemicals and reported heavy rooting from the treatment as compared to untreated cuttings. Solutions of indole-3-acetic acid were found to increase to a certain optimum concentration the number and length of roots per cutting as observed by Scholz (24). We found that cuttings of
R. dauricum sempervirens
Sims. treated by immersion for 24 hours at concentrations of 50 to 100 milligrams per liter resulted in 33 per cent rooting; not any of the untreated cuttings showed indications of root development. In using this treatment he stated that the optimum concentration was found different according to species or varieties and probably according to the maturity stage of the shoots used for propagation. Beta-indoleacetic acid solution when used in similar manner by Cox and Stoker (7) gave fatal results to cuttings of
R. lutescens
and
R. myrtilloides
within 22 days. Kirkpatrick (18) and Skinner (26,28) used various Catawbiense hybrids and
R. ponticum
and found the treatment using 40 to 100 milligrams per liter of indolebutyric or indoleacetic acids was advantageous in promoting root formation.
Investigating the possibility that amino acids might be a limiting factor, Doak (9) demonstrated that after pre-treating the cuttings of
R. maddenii jenkinsii
with alpha-naphthaleneacetic acid by solution immersion for 24 hours, 80 milligrams per liter, the application of an eight-day treatment by immersion in solutions of such compounds as lycine, histidien, and leucine increased rooting percentage in every case. These amino acids when applied in concentrations as low as 0.05 milligrams per liter were effective.
Leaf-bud cuttings were used by Skinner (27) who showed that cuttings of the variety 'Roseum Elegans', taken in June and treated with root promoting substances in liquid or powder forms, were 100 per cent rooted in 12 weeks; cuttings of R.
maximum
taken in July and treated with 90 milligrams per liter of indolebutyric acid by immersion for 24 hours were 100 per cent rooted within 13 weeks as compared to 20 per cent rooted for the untreated. It was suggested that interests in this method have centered around its value as a means of quickly reproducing such forms of the large-leaved hybrids which have been found difficult to propagate by other methods. Skinner gave evidence that a concentrated root promoting substance when applied to the cuttings, though producing a high percentage of rooted cuttings, may delay the production of shoot growth in that it was toxic to axillary buds. He believed that hormonized dusts were less toxic.
Using a different type of leaf-bud cutting, the leaf mallet, Doran (10) was able to show 50 per cent rooting with
R. maximum
, and Kirkpatrick (18) reported in agreement that this type was superior to terminal cuttings.
Approaching the study from environmental standpoints, Skinner (2 7 ) was the first to record that a day length of 18 hours significantly promoted root formation. A 20 per cent increase in rooting was obtained when such treatment was given cuttings of
R. catawbiense
'Album Elegans'.
A propagation study by Nearing and Connor (21 ), beginning in 1924 and lasting for 15 years, developed a method which was dependent upon using a stratified rooting medium and a special type of propagation frame that would provide adequate amount of north light. Using this propagation frame they showed 50 per cent rooting, at the end of 6 months, of cuttings taken in summer. Chadwick and Gunesch (5) concluded that a rooting medium of half sand and half peat was better than peat alone; and if sand alone was used, it should be of acid reaction.
A further change in environmental conditions was investigated by Eckstein (11) who studied the effects of increased oxygen concentrations in the rooting media. Using 70 per cent oxygen for the first 4 weeks and 50 per cent for the succeeding 6 weeks, he concluded that root primordia were not stimulated by this treatment.
Considering the factors of inhibition to rooting from an anatomical study, Eckstein (11) showed that in the young stem of
R. maximum
L. the cortex tissue was thick and was of a spongy, mesophyll-like structure. He reported that this type of structure might be a causal factor in slow rooting.
Wells (34) described a method whereby the bark of the cutting was slit vertically for a distance of ½ inches upwards from the base. The cuttings were taken at the junction of the current season's growth with that of the previous year. This was in agreement with Kemp (17) who claimed that at this point the diameter of the pith was usually smaller, and the presence of the meristem associated with the bud scale traces gave this location superiority. Knight (18) obtained good results in per cent rooting by cuttings of young twigs with a slight heel of older wood.
Another study of wounding the cuttings was considered by Kruyt (20) who reported cuttings scrapped to the cambium on one side at the base and then subjected to root promoting substances gave a higher percentage of rooting and a better developed root system than untreated cuttings. Upon working with
R. carolinianum
and several varieties of
R. catawbiense
he found that in each case the wound treatment gave heavier root systems than unwounded, and treatment with synthetic auxins increased rooting by 30 to 40 per cent. When the bark was removed for a distance of 1 inch from the base, as performed by Eckstein (11), increased rooting response was noted.
A physiological study was made by Pridham (23) who suggested age or maturity of wood as being highly important. He treated stock plants in cold storage of 50 degrees Fahrenheit for different periods and then took leaf-bud cuttings. Using root promoting substances his results showed 96 per cent rooting as compared to 70 per cent for the control plants in the field. Hanger (14) used softwood cuttings before the stem had stiffened with maturity and suggested the cuttings be taken in early morning while the temperature was cool.
Skinner (27) observed that cuttings made in late winter gave a lower per cent rooting and the time required longer than cuttings made in summer. This was in agreement with work reported in the American Rhododendron Society Quarterly Bulletin (1) in which fully matured branches were found to need double time for rooting; such cuttings were observed to need more than a year to root. Succulent to semi-mature wood has generally been considered superior to fully mature wood by other workers (17, 19).
Kemp (17) expressed the opinion that terminal shoots with vegetative buds were superior to those having flower buds, and the cut should be made close to a leaf. (1). Kruyt (20) observed evidence that cuttings from plants grown in shade produced roots in a shorter period than those grown in full sun. He further reported that thin crooked basal cuttings from side of plant gave heavier rooting than vigorous upright shoots.
Dipping leaves of cuttings to the petiole with Dowax, 1 to 3 dilution, before inserting them in the rooting medium adversely affected rooting as recognized by Skinner (27). In 5 or 6 weeks the cuttings became somewhat yellow which indicated the wax to be toxic or to interfere with the normal functioning of the leaf.
Materials And Methods
Investigations to determine the causal factors in slow and poor rooting of the
Rhododendron
were embarked upon from chemical, environmental, anatomical, and physiological studies.
Quantitative determinations of tannins were performed, and a group of cuttings was subjected to citric acid and wax treatments. The rooting response as influenced by environmental conditions of light, temperature, and humidity was investigated, and the effect of different oxygen concentrations in the atmosphere of the rooting medium was observed. Further experiments were carried out to learn the rooting response of cuttings when subjected to various wound and chemical treatments.
A study was made of the structure of the stem at different ages of growth, and respiration of stem portions and moisture uptake of wounded and unwounded cuttings were noted.

Chemical Studies
(1) Quantitative Analysis of Tannins . A blackening of the lower 1 to 2 inches of the cuttings was frequently observed, and a typical example is shown in Fig. 27. The cause of this blackening was believed to be due to oxidation of tannins present in the cutting. A suggestion by Eckstein (11) that tannins might be an inhibiting factor in rooting of rhododendrons was studied by chemical analysis. Since observations have been made that cuttings of certain species and varieties produce heavier root systems than others, a quantitative study of tannins seemed necessary in order to discover a possible correlation between difficulties of rooting and amount of tannin present.
Samples for the analysis were collected from the following species and varieties:
R. carolinianum
Rehd.
R. catawbiense
Michx.
R. catawbiense
variety
'Album Elegans'
'Duchess of Edinburgh'
'Gomer Waterer'
'Roseum Elegans'
R. fortunei
Lindl.
R. maximum
L.
R. maximum
variety
roseum
R. ponticum
L.
R. 'Watereri' Wils.
Care was taken to obtain a good representative sample. Collections, with the exception of R. 'Watereri', came from College Park and vicinity where the specimen plants had been subjected to the same environmental conditions and were taken during the months of March and April, 1951.
A quantitative study was made separately of the leaf, the petiole, the bark, and the wood; in further studies a comparison was made of vegetative and flower buds and 1 and 2 year stems.
Material was prepared for analysis and dried for 8 to 10 hours at 80 degrees Centigrade. Upon drying a 5 gram sample was obtained, and the tannin was extracted by boiling according to the procedure as outlined by the Association of Official Agricultural Chemists (2). After tannin was extracted, it was filtered and diluted to the recommended volume.
Quantitative analysis was performed by using the Loewenthal-Proctor titration method as described by Scott (25). This procedure consisted of the direct titration to a definite end point with potassium permanganate in the presence of indigo carmine indicator. Since solutions of tannin matters contain other oxidizable matter besides tannins, a separation of tannins was made by precipitation using gelatin and acidified sodium chloride, and a second titration was accomplished in order to ascertain the quantity of the permanganate actually required by the tannins. The difference between the oxidation values before and after the precipitation was taken as a measure of tannins present.
Calculations were made from these titrations, and the amount of tannins expressed in per cent dry weight was determined. Results are presented in Table I.
|
TABLE I. Tannin Content of Various Rhododendrons Expressed in Per Cent Dry Weight
(Samples taken March and April, 1951) |
|||||||
| TANNIN CONTENT 1 | |||||||
| Name of Plant | Leaf | Petiole | Stem 2 | Bark | Wood | Vegetative | Flower |
| 1 yr 2 yr | 1 yr 2 yr | 1 yr 2 yr | Bud | Bud | |||
| R. carolinianum | 3.93 | 3.21 | 5.72 _ 3 | 5.01 _ | 0.71 _ | _ | _ |
| R. catawbiense | 5.54 | 1.96 | 7.50 _ | 6.97 _ | 0.53 _ | _ | _ |
|
R. catawbiense
variety: |
|||||||
| Album Elegans | 8.33 | 2.50 | 7.55 _ | 7.02 _ | 0.53 _ | _ | _ |
| Duchess of Edinburgh | 7.68 | 6.79 | 8.40 _ | 7.33 _ | 1.07 _ | _ | _ |
| Gomer Waterer | 9.01 | 8.77 | 10.00 _ | 8.93 _ | 1.07 _ | _ | _ |
| Roseum Elegans | 7.50 | 2.86 | 7.35 _ | 4.64 _ | 0.71 _ | _ | _ |
| R. fortunei | 5.01 | 2.50 | 8.70 _ | 7.68 _ | 1.02 _ | _ | _ |
| R. ponticum | 4.80 | 2.86 | 5.89 _ | 5.18 _ | 0.71 _ | _ | _ |
| R. maximum | 5.18 | 3.39 | 9.46 3.89 | 7.86 3.55 | 1.60 0.34 | 2.50 | 2.86 |
| R. maximum roseum | 9.09 | 7.12 | 12.28 5.49 | 10.39 4.57 | 1.89 0.92 | 3.56 | 3.21 |
| R. Watereri | 5.36 | 4.29 | 5.72 _ 3 | 5.01 _ | 0.71 _ | _ | _ |
| Average | 6.49 | 4.20 | 8.05 4.69 | 6.91 4.06 | 0.95 0.63 | 3.03 | 3.04 |
|
1
Based on average of 3 determinations.
2 Content of bark and wood. 3 No determinations made.
|
(2) Chemical Treatment with Citric Acid and Wax
Chemical approach was made using citric acid and a wax emulsion to prevent the discoloration previously described. The citric acid, USP grade, was used in the crystalline form. The wax used was "Brytene" (Franklin Research Company, Philadelphia, Pennsylvania), diluted in a ratio of two parts wax to one part of water.
The procedure for treatment consisted of dipping the basal ends of the cuttings to a depth of approximately 1½ inches into the citric acid, and this was followed by a similar dip into the wax solution. These treatments were carried out in combination with various wound treatments.
One method of wounding was used whereby the bark at the base of the cutting was slit vertically for a distance of ½ inches upward. A second type of wound consisted of removing the bark for a distance of 1 inch from the base of the stem; and another procedure, to be used in later investigations, was that of making 3 upward slices, each ½ inch in length and ½ inch apart, into the bark. These wound treatments are shown in Fig. 28 and are referred to throughout the investigations as slit, stripped, and sliced.
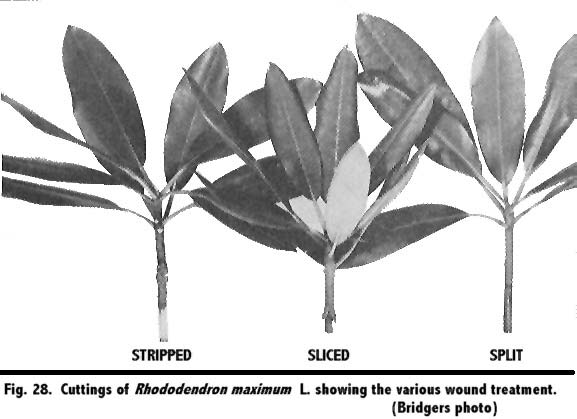
Cuttings of R. 'Watereri' Wils. were received from the Arnold Arboretum, Jamaica Plain, Massachusetts, on December 18, 1950. The following treatments were made using 30 cuttings per treatment.
1. Unwounded: Untreated
2. Unwounded: Citric Acid
3. Unwounded: Citric Acid, then waxed
4. Slit: Untreated
5. Slit: Citric Acid
6. Slit: Citric Acid, then waxed
7. Stripped: Untreated
8. Stripped: Citric Acid
9. Stripped: Citric Acid, then waxed
The cuttings were inserted into a rooting medium of half sand and half peat in a porous greenhouse bench with bottom heat controlled at 75 degrees Fahrenheit. Air temperature during the rooting period was approximately 70 degrees Fahrenheit, and relative humidity was from 60 to 70 per cent.
Results were based on per cent discoloration at basal ends of cuttings, average rooting score, and per cent rooting. To obtain the average rooting score for each treatment, cuttings were graded into 5 groups as shown in the following list and given the corresponding score.
1. Living but not callused, 1 point
2. Callused but not rooted, 2 points
3. Lightly rooted, 3 points
4.
Medium rooted, 4 points
5. Heavily rooted, 5 points
The average score was obtained by dividing the sum of the individual scores by the initial number of cuttings used in the treatment. This method was followed throughout the investigation, and heaviness of rooting and average rooting score should be considered synonymous.
Final data, recorded in terms of per cent discoloration at the basal end of the cuttings, average rooting score, and per cent rooting, are presented in Table II.
|
TABLE II. The Discoloration at Basal Ends and Rooting Response of Cuttings of
Rhododendron Watereri Wils. when Subjected to Citric Acid and Wax Treatments (Cuttings taken December 18, 1950; final data recorded February 16, 1951) |
||||
| Treatment |
Number
of Cuttings |
Per Cent
Discoloration of Basal Ends of Cuttings |
Average
Rooting Score |
Per Cent
Rooting |
| Unwounded: | ||||
| Untreated | 30 | 23.3 | 1.0 | 3.3 |
| Citric Acid | 30 | 43.3 | 1.0 | 0 |
| Citric Acid, then Waxed | 30 | 50.0 | 1.0 | 0 |
| Slit: | ||||
| Untreated | 30 | 50.0 | 1.0 | 0 |
| Citric Acid | 30 | 60.0 | 0.8 | 3.3 |
| Citric Acid, then Waxed | 30 | 50.0 | 0.8 | 0 |
| Stripped: | ||||
| Untreated | 30 | 73.3 | 1.0 | 3.3 |
| Citric Acid | 30 | 60.0 | 0.4 | 0 |
| Citric Acid, then Waxed | 30 | 56.7 | 1.2 | 6.7 |
Environmental Studies
(1) Rooting Response of Cuttings Subjected to Controlled and Changing Environmental Conditions of Temperature, Humidity, and Light
Environmental factors of light. temperature, and humidity have long been considered important in propagation of cuttings (30, 32). The design of this experiment was to show the effect of such conditions when controlled and when allowed to become variable.
For the controlled conditions a refrigerated room was provided with light of approximately 26 foot candles maintained by two fluorescent-type fixtures, each bearing forty watt daylight bulbs. These lights were hung at a distance of 2 feet above the tops of the cuttings as recommended by Stoutemyer and Close (31) , and a day length of 18 hours, as used by Skinner (27), was maintained. Temperature was held at 50 degrees Fahrenheit, while the relative humidity as measured by a psychrometer was maintained at 90 to 98 per cent. Bottom heat of 80 degrees Fahrenheit was controlled.
To study the rooting response as influenced by variable conditions of temperature, humidity, and light a raised bench in the greenhouse was used. Bottom heat of 80 degrees was provided as above. The greenhouse had a partial shade, but temperature and humidity were not controlled. In April and May a day temperature of 90 degrees was often observed, and in June a temperature of 100 degrees was recorded; a night temperature of 68 to 70 degrees prevailed. Relative humidity ranged from 30 to 60 per cent. No artificial light was provided, and the normal day length for this area during the months of the investigation varied from 12 to 16 hours.
Cuttings of
R. maximum roseum
were received from the United States Department of Agriculture Plant Introduction Gardens, Glenn Dale, Maryland, on April 18, 1951. In this study the stripped and sliced wound treatments were used in combination with Hormodin 3 and Fermate, prepared in a ratio of 3 to 1, with and without wax.
After the wound treatment was made the cuttings were dipped into the Hormodin 3 and Fermate mixture as outlined by Zimmerman and Hitchcock (36); when the wax was used the treatment was performed as previously described.
Four groups of 20 cuttings each were prepared and subjected to the following treatments.
1. Stripped: Hormodin 3 plus Fermate (3:1)
2. Stripped: Hormodin 3 plus Fermate (3:1), then Waxed
3. Sliced: Hormodin 3 plus Fermate (3:1)
4. Sliced: Hormodin 3 plus Fermate (3:1), then Waxed
After treatment the cuttings were inserted in a rooting medium of half sand and half peat in the controlled and greenhouse propagation benches.
Final data, presented as average rooting score and per cent rooting, are shown in Table III.
| TABLE III. Rooting Response of Rhododendron maximum roseum Cuttings as Influenced by Controlled and Changing Environmental Conditions of Temperature, Humidity, and Light (Cuttings taken April 18, 1951; final data recorded June 15, 1951) | ||||
| Environment | Treatment |
Number
of Cuttings |
Average
Rooting Score |
Per Cent
Rooting |
|
Controlled
(in refrigerator) |
Stripped: | |||
| Hormodin 3 plus Fermate (3:1) | 20 | 2.1 | 30 | |
| Hormodin 3 plus Fermate (3:1), then Waxed | 20 | 2.0 | 30 | |
| Sliced: | ||||
| Hormodin 3 plus Fermate (3:1) | 20 | 2.3 | 50 | |
| Hormodin 3 plus Fermate (3:1), then Waxed | 20 | 2.1 | 45 | |
|
Uncontrolled
(in greenhouse) |
Stripped: | |||
| Hormodin 3 plus Fermate (3:1) | 20 | 2.1 | 35 | |
| Hormodin 3 plus Fermate (3:1), then Waxed | 20 | 2.0 | 25 | |
| Sliced: | ||||
| Hormodin 3 plus Fermate (3:1) | 20 | 0.6 | 5 | |
| Hormodin 3 plus Fermate (3:1), then Waxed | 20 | 0.5 | 0 | |
The flow of oxygen and nitrogen gases entered the rooting medium from a gas chamber of the exact size of the propagating pan and located just below the medium. A metal screen of ¼ inch mesh and a layer of pea gravel were used to separate the chamber from the medium. The gas entered the chamber through a plastic tube, ⅜ inch in diameter, which was arranged to give a uniform concentration. There was a constant diffusion upward through the pea gravel and into the rooting medium.
A regulator valve and a Y connector attached to the oxygen and nitrogen bottles served as a means of regulating the gas flow. The gas was bubbled through water contained in a 500 milliliter wide mouth glass bottle, a process which served to regulate the flow and to prevent dehydration of the medium by the gases.
In order to hold constant the desired oxygen concentrations, it was found necessary to place a seal over the medium. This was done by using a cardboard paper, with holes punched for inserting the cuttings. The paper was then treated with a tree asphalt paint which made it impervious to gas. After the cuttings were inserted through the paper and into the medium, a small portion of the paint was used to seal them in place. There were openings in the seal to provide gas outlets. The normal atmosphere pan was unsealed, and the cuttings were inserted in the usual manner.
Water was by sub-irrigation and was performed once a week. Within an hour after watering, the atmosphere of the medium was again controlled.
Gas analyses for oxygen and nitrogen were made at regular intervals with the Fisher-Orsat apparatus. Using; capillary glass tubing, samples of gas were taken from the gas chamber and also from the vicinity of the basal ends of the cuttings.
For this experiment cuttings of
R. maximum roseum
were obtained from the United States Department of Agriculture Plant Introduction Gardens, Glenn Dale, Maryland, on April 18, 1951. Fifty cuttings for each pan were prepared and subjected to the following treatments.
1.
Stripped
: Hormodin 3 plus Fermate (3:1)
2.
Stripped
: Hormodin 3 plus Fermate (3:1), then Waxed
On June 15, 1951, the cuttings were removed from the bench, and final observations were made according to per cent and heaviness of rooting. Results are presented in Table IV.
| TABLE IV. The Influence of Changing the Atmosphere of the Rooting Medium on the Rooting Response of Rhododendron maximum roseum (Cuttings taken April 18, 1951; final data recorded June 15, 1951). | ||||
|
Atmosphere
of Rooting Medium |
Treatment |
Number
of Cuttings |
Average
Rooting Score |
Per Cent
Rooting |
|
High
Oxygen Content (36-40%) |
Stripped: | |||
| Hormodin 3 plus Fermate (3:1) | 25 | 1.4 | 40 | |
| Hormodin 3 plus Fermate (3:1), then Waxed | 25 | 2.0 | 40 | |
|
Normal
Oxygen Content (18-20%) |
Stripped: | |||
| Hormodin 3 plus Fermate (3:1) | 25 | 1.9 | 32 | |
| Hormodin 3 plus Fermate (3:1), then Waxed | 25 | 1.7 | 25 | |
|
Low
Oxygen Content (4-7%) |
Stripped: | |||
| Hormodin 3 plus Fermate (3:1) | 25 | 0.4 | 4 | |
| Hormodin 3 plus Fermate (3:1), then Waxed | 25 | 0.3 | 0 | |
Anatomical Studies
Sections of the stem were prepared for microscopic study by imbedding the material in parlodion. The procedure recommended by Johansen (16) was used.
Stem portions of
R. maximum
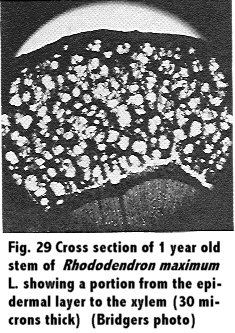
L. were obtained on October 22, 1950, from College Park, Maryland, and were cut ¼ inch in length. They were placed in a killing and fixing solution of formalic-aceto-alcohol, and this was followed by dehydration with alcohol. The material was imbedded, and sections 30 microns thick were obtained using the sliding microtone. Stain was the safrannin-fast green combination.
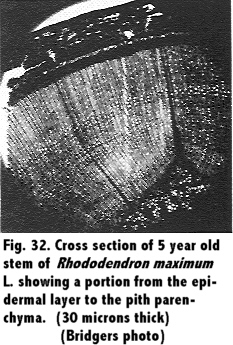
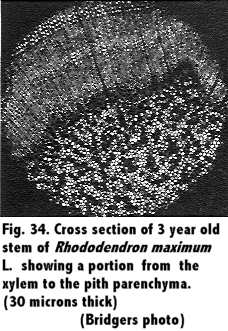
A study of the stem in cross section was observed, and comparisons between different ages of wood were made. Results are shown in Fig. 29 through Fig. 34.
|

|

|
|

|
|
Combination Wound And Chemical Treatments
The anatomical study indicated the stem to have a heavily cutinized epidermal layer, and it was believed that this might be a possible factor in causing slow root initiation. In order to eliminate this factor, cuttings were subjected to a series of wound treatments, previously described, and the effect of these treatments with certain root promoting substances was noted.
(1) Rooting Response of Cuttings as Influenced by Initial Wound Treatment and Corresponding Wound Retreatment with Hormodin 3.
For a preliminary investigation to determine the effect of certain wound treatments when used alone, cuttings of catawbiense varieties of Duchess of Edinburgh, Gomer Waterer, and Roseum Elegans were obtained Au-List 10, 1950, from A. Gude and Sons Nursery, Rockville, Maryland. Each of these varieties was divided into three groups of 48 cuttings each and given the following treatments.
1. Unwounded
2. Slit
3. Stripped
After treatment the cuttings were inserted in a medium of half sand and half peat in the controlled refrigerator room previously described.
At the end of 12 weeks the cuttings were removed from the medium. It was observed that roots were not developing, and more than 85 per cent of the cuttings had a severe discoloration at the base for a distance of 1 to 2 inches.
These discolored areas were removed, and the cuttings were retreated with the respective wound treatments. At this time all of the cuttings were treated with Hormodin 3 as outlined by Zimmerman and Hitchcock (36) and reinserted in the rooting medium.
Final records were based on per cent rooting and heaviness of rooting.
( 2) Rooting Response as Influenced by Age of Wood, Presence of Flower Buds, and Various Wound and Chemical Treatments.
It has been suggested that flower buds may be detrimental to root initiation and development in rhododendron cuttings (17) and that maturity of wood might be a factor (1, 14, 17, 19, 23, 27). To investigate these factors an experiment was designed to learn the effect of wound treatment in combination with certain chemical treatments upon the rooting response of shoots having flower buds and those having vegetative buds. A comparison of the effect of such treatments on different ages of wood was also made.
To carry out these investigations cuttings of
R. maximum roseum
were secured from the United States Department of Agriculture Plant Introduction Gardens, Glenn Dale, Maryland, on November 12, 1950. The cuttings were selected according to type and given the following treatments.
| With Flower Buds (12 cuttings per treatment) | |
|
a. Cut at junction of 1 and 2 year wood
1. Unwounded: Untreated 2. Stripped: Untreated 3. Stripped: Waxed |
b. Two year wood
|
| Without Flower Buds (15 cuttings per treatment) | |
|
a. Cut at junction of 1 and 2 year wood
1. Unwounded: Untreated 2. Stripped: Untreated 3. Stripped: Waxed 4. Stripped: Hormodin 3 5. Stripped: Hormodin 3, then Waxed 6. Stripped: Hormodin 3 plus Fermate (3:1) 7. Stripped: Hormodin 3 plus Fermate (3:1), then Waxed |
b. Two year wood
1. Unwounded: Untreated 2. Stripped: Untreated 3. Stripped: Waxed 4. Stripped: Hormodin 3 5. Stripped: Hormodin 3, then Waxed 6. Stripped: Hormodin 3 plus Fermate (3:1) 7. Stripped: Hormodin 3 plus Fermate (3:1), then Waxed |
After treatment the cuttings were inserted in a propagation bench in the greenhouse and were subjected to the same environmental conditions as previously described.
(3) Rooting Response as Influenced by Various Wound and Chemical Treatments.
This experiment was designed to compare various wound treatments when the cuttings were treated with a series of root promoting substances, with or without wax. Shoots with vegetative buds were used and were cut at approximately 11/a inches above the junction of the current season's growth and that of the previous year.
Cuttings of R. 'Watereri' Wils., received from the Arnold Arboretum, Jamaica Plain, Massachusetts, were prepared on February 18, 1951. Treatments, with 18 cuttings in each, consisted of the following.
1. Slit: Hormodin 3 plus Fermate (3:1)
2. Slit: Hormodin 3 plus Fermate (3:1), then Waxed
3. Slit: Untreated
4. Slit: Indolebutyric Acid (100 milligrams per liter, solution immersion for 24 hours)
5. Slit: Indolebutyric Acid (100 milligrams per liter, solution immersion for 24 hours), then Waxed
6. Stripped: Hormodin 3 plus Fermate (3:1)
7. Stripped: Hormodin 3 plus Fermate (3:1), then Waxed
8. Stripped: Untreated
9. Stripped: Indolebutyric Acid (100 milligrams per liter, solution immersion for 24 hours)
10. Stripped: Indolebutyric Acid (100 milligrams per liter, solution immersion for 24 hours), then Waxed
11. Sliced: Hormodin 3 plus Fermate (3:1)
12. Sliced: Hormodin 3 plus Fermate (3:1), then Waxed
13. Sliced: untreated
14. Sliced: Indolebutyric Acid (100 milligrams per liter, solution immersion for 24 hours)
15. Sliced: Indolebutyric Acid (100 milligrams per liter, solution immersion for 24 hours), then Waxed
After treatment the cuttings, thrice replicated, were inserted in a propagation bench in the greenhouse. The same environmental conditions, previously described, prevailed.
On June 1, 1951, the cuttings were removed from the bench, and results were recorded in terms of per cent o£ cuttings rooting and average rooting score. The data were subjected to analysis of variance as recommended by Goulden (13).
Observations indicated that the sliced and stripped wound treatments produced a higher per cent rooting and earlier rooting than the slit wound treatment. Further comparisons of the sliced and stripped treatments were made in order that the best treatment might be determined.
For this study cuttings of
R. catawbiense
'Roseum Elegans' were obtained from the Morris Arboretum, Philadelphia, Pennsylvania, on March 21, 1951. Groups of 30 cuttings each were prepared and given the following treatments.
1. Sliced: Hormodin 3 plus Fermate (3:1)
2. Sliced: Hormodin 3 plus Fermate (3:1), then Waxed
3. Sliced: Untreated
4. Sliced: Indolebutyric Acid (100 milligrams per liter, solution immersion for 24 hours)
5. Sliced: Indolebutyric Acid (100 milligrams per liter, solution immersion for 24 hours), then Waxed
6. Stripped: Hormodin 3 plus Fermate (3:1)
7. Stripped: Hormodin 3 plus Fermate (3:1), then Waxed
8. Stripped: Untreated
9. Stripped: Indolebutyric Acid (100 milligrams per liter, solution immersion for 24 hours)
10. Stripped: Indolebutyric Acid (100 milligrams per liter, solution immersion for 24 hours, then Waxed
After treatment the cuttings, thrice replicated, were placed in a greenhouse propagation bench and were subjected to similar environmental conditions as previously described.
Final data, in terms of per cent rooting and average rooting score, were subjected to analysis of variance as outlined by Goulden (13).
Physiological Studies
(1) Moisture Uptake of Wounded and Waxed Cuttings.
This investigation was performed in search for a possible explanation as to the reason wounded cuttings rooted heavier and at a higher per cent than unwounded cuttings. Day (8) suggested that this increase in rooting response might be due to a corresponding increase in water uptake by wounded cuttings. Wax was used in combination with the wound treatments in order to learn its effect upon water uptake.
For this study cuttings of
R. maximum
L. were obtained from College Park, Maryland, on June 10, 1951. Four groups of 18 cuttings each were prepared and subjected to the following treatments.
1. Unwounded
2. Slit
3. Sliced
4. Stripped
The cuttings were then arranged in groups of 3, making a total of 6 groups for each treatment, and were weighed. After the weights were recorded, 3 groups of each treatment were further treated with the wax, using the procedure previously described.
The cuttings, thrice replicated, were then inserted in a greenhouse propagation bench. Bottom heat of 75 degrees Fahrenheit was controlled. During the course of the investigation, day temperature was approximately 85 degrees, with a night temperature of 68 degrees. Relative humidity was seldom over 65 per cent.
After 36 hours the cuttings were removed, reweighed in respective groups, and the amount of moisture uptake calculated.
(2) Respiration Studies of Wounded and Waxed Stem Tissue.
Manometric methods for calculating exchange of gases have been in use in the study of both chemical and biological reactions for many years (33). The type of instrument used in this experiment to study the respiration of excised lengths of stems was the Warburg Respirometer.
For this investigation stems of
R. maximum
L. were obtained from College Park, Maryland, on July 3, 1951. They were cut into portions of approximately 1½ inches in length and given the following treatments.
1. Unwounded
2. Slit
3. Stripped
4. Sliced
Samples, prepared in duplicate, were weighed, using approximately 2½ grains per sample. One cut end of each stem portion was lightly sealed over with paraffin, while the duplicate was treated in the same manner and entirely waxed with "Brytene". The samples were placed in large flasks of approximately 80 milliliters in volume, to which had been added 3 milliliters of 20 per cent potassium hydroxide solution.
The flasks were connected to the respective manometers, and the conventional procedure was followed. Temperature of the water bath was controlled at 30 degrees Centigrade, and readings were made at intervals of 10 minutes.
By the formula of Umbriet (33) flasks constants were determined, and the relative amount of oxygen uptake calculated in microliters per 10 minutes is presented in Figures I and II.
Results And Discussion
Chemical Studies
The results of the quantitative analyses of tannins in various species and varieties of rhododendrons as shown in Table I, indicated that tannins are found in greatest concentration in 1 year old stems. This is followed, in descending order, by bark of 1 year stem, leaf, 2 year stem, petiole, 2 year bark, flower and vegetative buds, and wood. These observations were in accordance with the work of Clarke and Rogers (6) who found that in various species of
Rhus
the bark and leaves contained from 25 to 30 per cent more tannin than the wood.
This investigation was influenced by the possible correlation between the factors of rooting difficulty and amount of tannin present. Observing the data one could not formulate this correlation because cuttings of
R. maximum roseum
root readily, but according to the analyses the amount of tannin present in the 1 year stem was more than 4 per cent higher than the average obtained from the species and varieties tested. Observations by the author, and Doran (10) reported in agreement, have indicated the cuttings of
R. maximum
root slowly, yet this species has a tannin content of 9.46 per cent in the 1 year stem as compared to 12.28 per cent for the variety roseum.
Based on averages obtained in the analyses it was noted that tannin content of 1 year old stem to be 8.05 per cent, whereas that of the 2 year stem was 4.69 per cent. Breaking the stem down into component parts, namely the bark and wood, there was found a tannin content of 6.91 per cent in the 1 year bark as compared to 4.06 per cent in that of the 2 year bark. There was noticed a decrease in tannin content of 2 year wood as compared to that of 1 year wood. The explanation of this was believed to be in the maturation of 2 year stems. Tannin content, therefore, did not appear to be a reason for the decrease in rooting response observed in cuttings made from 2 year growth. No positive explanation was obtained for the apparent detrimental effect of flower buds as there was no appreciable difference in tannin content of flower and vegetative buds tested.
A high tannin content did not appear to be associated with ease of rooting because the variety Roseum Elegans has been considered to root easily yet the tannin content was lower than that of R. maximum, a difficult species to root.
A study approaching the discoloration at the basal ends of cuttings using citric acid and wax treatments indicated, as shown in Table II, that when the cuttings were unwounded or slit, these treatments did not prove beneficial. However, in the stripped treatment, citric acid, with or without the wax, decreased the per cent of discoloration from 73.3 to 56.7. The discoloration appeared to be greater in wounded cuttings than in unwounded.
Instead of an oxidation of tannins being the cause of the discoloration, the cause was believed to be due to cell injury by the mechanical wound. Bloch (3) discussed that cells in such wound regions might degenerate and die, and their content might undergo further post mortal changes which probably would effect the metabolism of adjacent healthy cells. Kemp (17) noticed a blackening from the base upwards in cuttings of many woody plants and gave as an explanation an oxidation process of the tissue brought about by delayed healing. He was of the opinion that a crushed and mutilated, instead of a cleanly cut wound, caused greater discoloration.
A further study of the data showed that based on rooting response the treatment using citric acid, with or without wax, did not stimulate rooting to be of practical use.
Environmental Studies
Possible factors of inhibition to rooting of rhododendron cuttings were considered by environmental studies. For many years the plant propagator has considered controlled environmental conditions for difficult-to-root plants and Stoutemyer (30) concluded that wide fluctuations in temperature, which take place in most propagating structures in spring and summer. resulting in sudden changes in humidity, caused desiccation and loss of turgor pressure in plants and was a possible inhibition factor to rooting.
Data, shown in Table III, have indicated that cuttings of
R. maximum roseum
subjected to the sliced wound treatment in combination with Hormodin 3 and Fermate (3:1), with or without wax, and placed in the refrigerated room of controlled conditions of temperature, humidity, and light, rooted more heavily and in higher per cent than the cuttings subjected to the same treatment but rooted under uncontrolled conditions. When cuttings subjected to the stripped wound treatment, and given the same chemical treatment as described above, there was little difference in rooting response obtained from the controlled and uncontrolled conditions.
These results have not been substantiated by reviewed literature, and the author has no explanation.
When cuttings of
R. maximum roseum
were placed in a rooting medium having an atmosphere enriched with an oxygen content to approximately 38 per cent, there (Table IV) was a decided increase in per cent rooting over those cuttings placed in a normal medium atmosphere with an oxygen concentration of 18 to 20 per cent. A low oxygen content showed a detrimental effect on heaviness of rooting and in per cent rooting.
Zimmerman (35) reported that oxygen has often been found to be a limiting factor in root initiation of cuttings of many plants. He has observed
1
that root initiation in rhododendron cuttings occurred in the cambium, and he was of the opinion that root formation would be inhibited by a decrease in oxygen. Conversely, rooting would be stimulated by increased oxygen concentrations.
1 Private Communications-Dr. P. W. Zimmerman, Boyce Thompson Inst. of Plant Research, Yonkers, N. Y. March, 1951.
Anatomical Studies
A study of the anatomy of the stem of the Rhododendron in cross section, Fig. 29 through 34, showed the tissues to the outside of the cambium to be spongy, and in the young stem the arrangement was similar to mesophyll cells of a leaf. This was in agreement with Eckstein (11) who reported of a similar structure. These tissues of a 1 year old stem showed a much greater size in proportion to the xylem than in older twigs, a range from a 2 to 1 ratio in 1 year stems to a 1 to 4 ratio in a 5 year stem.
Observations showed the xylem to be a diffuse porous with mostly uniseriate rays though with a few multi-seriate rays. Phloem appeared to be indistinguishable in cross section from the pericycle and the phelloderm, and close observation showed that the young stem had a very heavily cutinized epidermis.
The annual rings in the xylem were formed by the cessation of 1 year's growth and the small cells produced at that time and the formation of large cells with the succeeding year's growth. Close observation showed that toward the end of the second year of growth sclerenchyma cells were produced in isolated groups in the phloem region. In addition, and at about the same time, a continuous row of sclerenchyma cells were produced in the vicinity of the same region. In subsequent growth periods other such rings or bands of sclerenchyma cells were produced by the cambium, and in a 5 year old twig there were 4 rows of such cells visible as shown in Fig. 32. The heavily cutinized epidermis was still present on the 5 year old twig.
According to the study of the stem structure little information concerning a possible inhibition to rooting due to stem anatomy was obtained. Kemp (17) stated that there was no satisfactory explanation as to why plants having thick stems were more difficult to root than the thin stemmed species. In rooting cuttings which he observed microscopically, not one had been found where a root had been prevented from emerging by structural features of the stem. These emerging roots were found to quickly push aside or rupture any obstruction.
It was believed by the author that the only possible factor in the stem structure which might cause root inhibition was the heavily cutinized epidermal layer. This cutin prevents water loss in plants, and it might inhibit moisture uptake by the stem cutting.
To be continued.