JARS v54n3 - Growing Rhododendrons on Limestone Soils: Is it Really Possible?
Growing Rhododendrons on Limestone Soils:
Is it Really Possible?
Anthony J. McAleese and David W. H. Rankin
Department of
Chemistry
University of Edinburgh
Edinburgh, Scotland, U. K.
Synopsis
Analyses of leaves and soil samples from the rooting zone of wild (Chinese) rhododendrons have confirmed that some species can grow in soils which contain high proportions of limestone and have high pH. Some healthy specimens show low iron and manganese concentrations in leaves, and deficiencies of these elements are possible causes of chlorosis in rhododendrons grown on alkaline soils. However, many rhododendrons and other ericaceous plants exhibit very high manganese content in their foliage, and foliar decay may play a major role in providing manganese for living plants.
Rhododendrons need acid soil. That seems to be a fundamental tenet of belief, although there is plenty of evidence that it is not always true. There have been many reports of Rhododendron species growing on or over limestone rocks in Western China (Kingdon Ward, 1926; Cox, 1945), of R. hirsutum L. on limestone in the European Alps (Cox and Cox, 1997), of R. occidentale (Torr & A. Gray) A. Gray on serpentine in Oregon (Leiser, 1957), and of R. prinophyllum (Small) Millais on neutral soils in Ohio (Widrlechner, Larson and Dragula, 1993), as well as studies of species such as R. micranthum Turcz. growing in containers on lime-supplemented media (Chaanin, 1998). But the strength of the belief that acid conditions are always essential has led to attempts to explain away these observations, the hypothetical explanations in time becoming assertions, that are then accepted as fact.
We have studied rhododendrons growing in the limestone mountains of North-West Yunnan, China [photograph 1], examining both the plants themselves and the soils in which they grow, as well as rock and water samples extracted from the root zones. We chose sites with soil pH* ranging from about 3.7 to 8.6, all with healthy rhododendrons. Indeed, we never found any Rhododendron plants showing symptoms of nutrient deficiency, with the exception of one which was on the edge of a quarry, and probably suffering from drought. Our investigations of the soils (McAleese etal., 1999) forced us to the conclusion that past "explanations"; for observations of rhododendrons growing on limestone are incorrect; that there are some rhododendrons which do grow in alkaline soil, with the roots in contact with limestone, available to the plants.
 |
 |
|
Photograph 1. The limestone mountains of Western China: the Yulong Shan in North-West Yunnan Province. |
Photograph 2. Rhododendron telmateium growing in limestone. The soil has been cut away to reveal the root zone. |
Commonly Held Theories of How Rhododendrons Grow on Limestone
Rhododendrons growing over limestone are in fact rooted in pockets or layers of organic material,
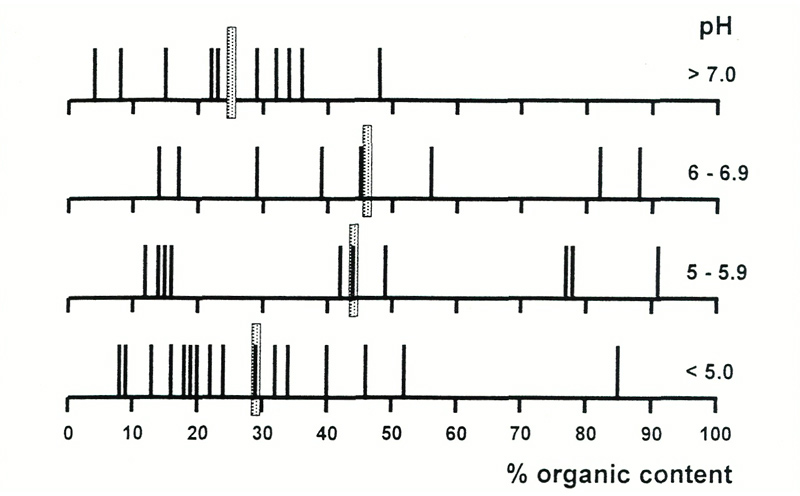
out of contact with the limestone (Grove, 1927). Figure 1 shows the percentage of organic content
in the soils, and indicates that few soils are predominantly organic, and that most soils are less
than 50% organic, regardless of their acidity or alkalinity. Moreover, analyses for calcium, which
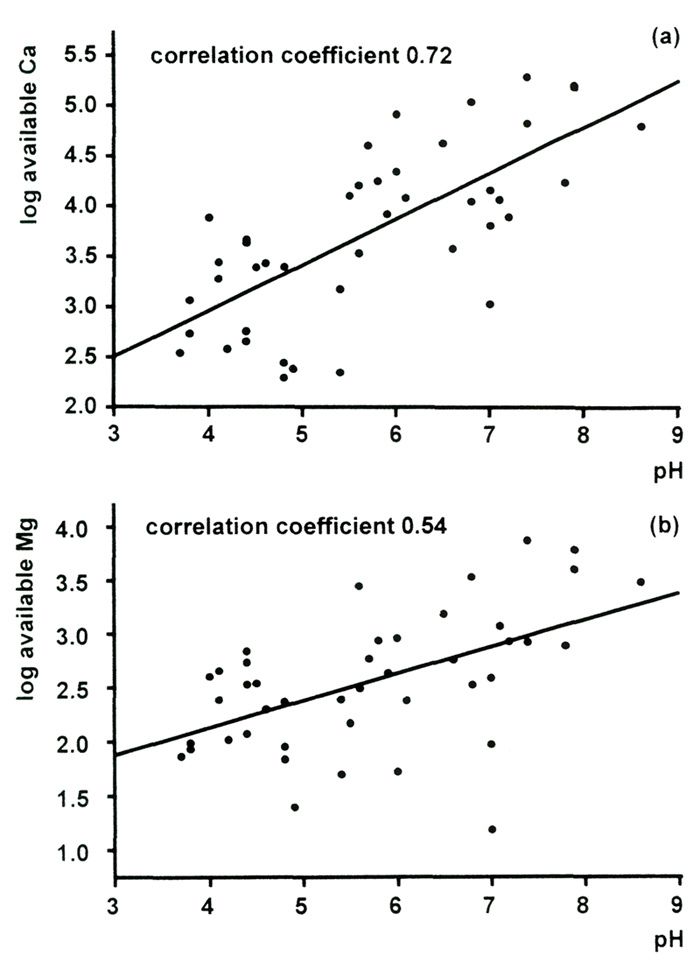
is the metal that is the predominant component of limestone (calcium carbonate), show that its
concentration correlates strongly with pH [Figure 2(a)].
These data confirm what is obvious by inspection; there are some Rhododendron plants which quite clearly have their roots in white, powdered limestone, with very little organic material present at all [photograph 2]. Of course, there are plenty of places where there is a thick layer of organic-rich material over the limestone, but this does not mean that such a layer is ubiquitous. We therefore reach by analysis the same conclusion that George Forrest reached in 1915, using only his eyes: "Most of the rhododendrons in that region (North-West Yunnan) grow directly in, or on, pure limestone" (Cox, 1945).
 |
|
Figure 1. Organic content of soils as a function of pH. Averages are shown by shaded bars,
individual measurements by black bars. Most soils in all pH ranges have a high non-organic content, i.e., of material derived from rock. |
Companion calciphile plants also confirm that some rhododendrons are happily adapted to very alkaline conditions. For example, Rhododendron telmateium Balf. f. W. W. Sm. and R. cuneatum W. W. Sm. grow alongside such confirmed lime-lovers as Primula forrestii and Daphne calcicola, while we have found R. primuliflorum Bureau Franch. and R. rupicola W. W. Sm. growing in limestone crevices shared with Paraquilegia anemonoides, another species associated uniquely with limestone.
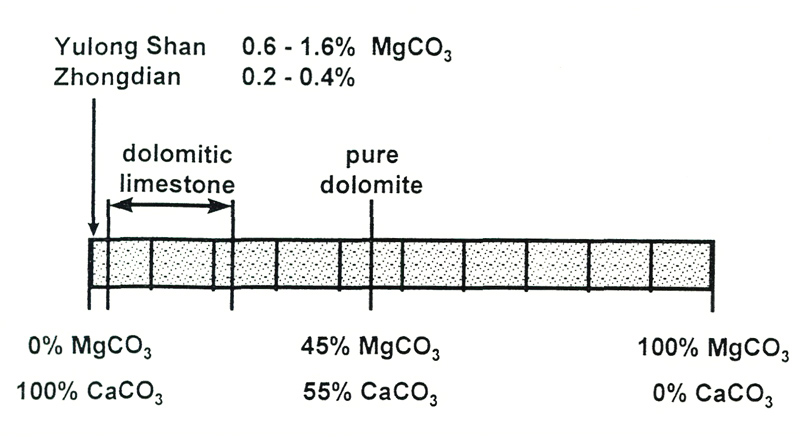
The limestone in which the rhododendrons grow is dolomitic (Hartge, 1956). This could be highly significant. Dolomite is a mixture of equal amounts of calcium carbonate and magnesium carbonate. Calcium is a bit heavier than magnesium, so equal proportions gives 45% magnesium carbonate by weight.
 |
|
Photograph 3. Rhododendron rupicola growing in limestone crevices. |
Dolomitic limestone is the term used for rock which has upwards of 2.5% magnesium carbonate. Magnesium is an essential part of chlorophyll, the green pigment in leaves which absorbs energy from sunlight and allows growth. The theory is that calcium, which is chemically similar, competes with the magnesium for uptake by the roots of the plants, and so hinders growth. However, our analyses show that the samples of limestone from Yunnan only have between 0.2 and 1.6% magnesium carbonate, so they are certainly not dolomitic (Figure 3). And as we shall see, a large excess of calcium does not inhibit take-up of magnesium anyway. The amount of available magnesium in our soil samples was generally about one-tenth of that of calcium, and increased with pH ([Figure 2(b)] - it certainly did not become less available in alkaline soils.
 |
|
Figure 2. Correlation of pH and concentrations of (a) available calcium and (b) available magnesium in Rhododendron root soils. |
The limestone is hard and more or less insoluble (Tod, 1971 a, 1971 b). Our geologist colleagues have labeled all the limestone specimens as soft, and that is consistent with their easy erosion, and particularly with their dispersion as fine particles in glacier melt-water. We have analyzed soils for what is known as "available" calcium, i.e., the part in the soil which is sufficiently soluble to be available for uptake by the plant roots. The data shown in Figure 2 confirm that the calcium carbonate is not locked up in hard, insoluble rock.
 |
|
Figure 3. Composition of dolomite and dolomitic limestones. The amount of magnesium
in limestone samples from North-West Yunnan is far too low for them to be classified as dolomitic. |
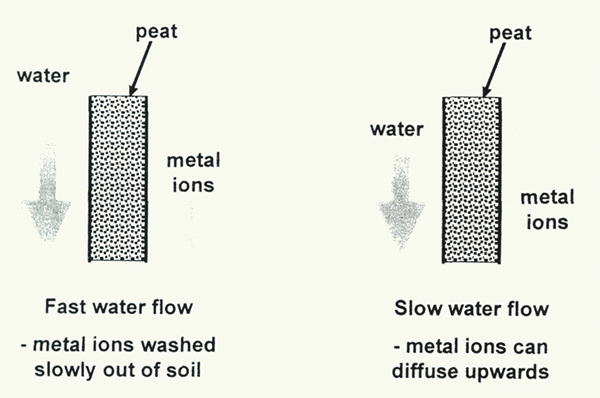
Persistent heavy rain in these monsoon-affected areas washes any dissolved limestone out of the soil (Hartge, 1956). There are three difficulties with this. First, as we have seen, the calcium is clearly present in the soil samples. Secondly, the idea of washing the dissolved material out of the soil is only possible if there is an organic layer above the limestone rock, and separate from it. We have shown that the organic layer is mixed with, and sometimes dominated by, the inorganic (rock-derived) component. Thirdly, the rain is not incessant; spring and fall are dry seasons, when moisture at the roots of rhododendrons comes primarily by capillary action from below, or may move slowly downwards after any rain. Either way, salts dissolved from the underlying rocks can diffuse upwards into the root zone (Figure 4).
 |
|
Figure 4. When a lot of water flows through peaty soil, metal salts are slowly
washed through (leached), although they are held quite tightly by the peat. When the water flow is low, diffusion of metal ions can be sufficient to transport salts upwards, against the flow of water. |
We cannot escape the conclusion that there are situations in which rhododendrons do grow with their roots in contact with available limestone, and that this does them no harm. So we have had to reject all of these theories. Perhaps we should not be surprised: there are plenty of other ericaceous plants which grow in alkaline soils. But we are left with the question, "Why do most rhododendrons in cultivation dislike limestone?" It cannot be that they are short of magnesium, nor that calcium in the soil is toxic to them. The latter has also been shown by careful cultivation experiments (Drehmel and Preil, 1992), although Tod (1956) assumed calcium toxicity to be significant, solely on the basis of an experiment involving soil with high pH induced by addition of magnesium carbonate. Nor can it be caused by the carbonate ions from the limestone (at high pH) or bicarbonate (at somewhat lower pH), because they are abundant in our soil samples.
Bicarbonate ion has been claimed to be significantly toxic to rhododendrons (Chaanin and Preil, 1994, 1996; Chaanin, 1998), but it should be noted that merely adding bicarbonate solutions to soil does not necessarily increase its concentration in the soil, because it is in equilibrium with atmospheric carbon dioxide; conversely, it will always be present in soil in the appropriate pH range (around pH 6), whether or not it has been specifically added as bicarbonate.
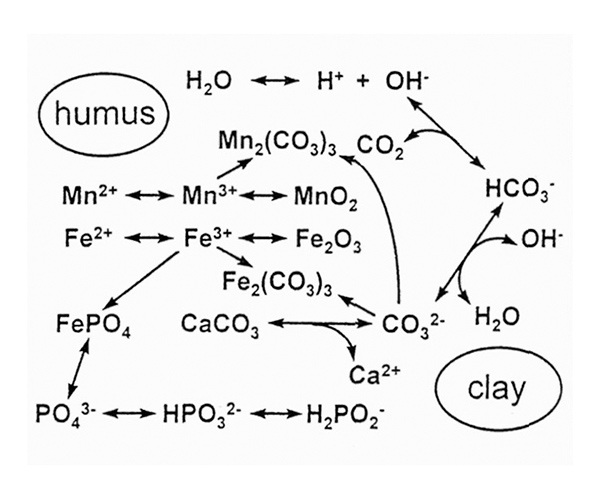
If all these more straightforward explanations of rhododendrons appearing to tolerate high pH soils in some wild sites fail, we must turn our attention to the foliage of the plants, and to iron and manganese, two other metals which are essential for plant growth and which must be obtained from the soil. However, we also need to recognize that concentrations of the relevant chemical species in solution are interdependent (Figure 5). Changing the concentration of any one constituent changes all the others as well, and this diagram shows only a tiny fraction of all the components that are involved. Moreover, concentrations in aqueous solution in the soil are not the same as those in or on the surface of soil solids. Organic and inorganic materials interact in different ways with different ions, so that the whole system is extremely complicated.
 |
|
Figure 5. Concentrations of compounds and ions important to plants are linked by a
series of inter-related equilibria. Concentrations within organic and non-organic solids in the soil will be quite different from those in aqueous solution. |
Results and Discussion
CALCIUM AND MAGNESIUM IN THE FOLIAGE
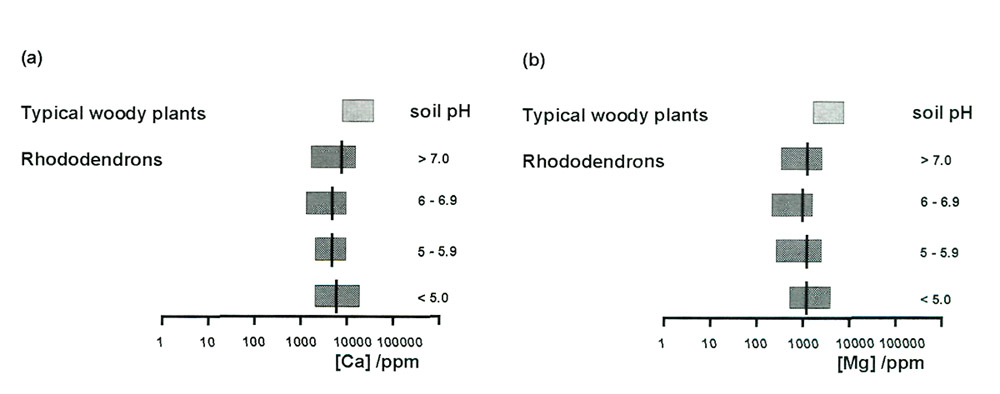
Measurements of calcium concentration in the leaves of rhododendrons confirm their remarkable
tolerance of a huge range of calcium concentrations in the soil. Figure 2(a) showed that
there was a factor of 1000 between the highest and lowest available amounts of calcium in the
sample soils, and that this concentration correlated strongly with soil pH. Figure 6(a) displays
the concentration ranges of calcium in the leaves of rhododendrons, grouped according to the
pH of the soils in which they were growing, and shows that there is no correlation with pH at all.
There is a ratio of only 15 between the highest and lowest, for 80 leaves from 80 separate plants
on 40 sites, so uptake of calcium is clearly well controlled, and the insensitivity of plants to
available soil calcium is demonstrated. Much the same applies to magnesium. The soil concentrations
[Figure 2(b)] were about a factor of 10 lower than those of calcium, and the same is true for the
leaf concentrations [Figure 6(b)]. In both cases the observed ranges for rhododendrons are somewhat
lower than is typical for woody plants, as has been noted previously (Tod, 1971b).
 |
|
Figure 6. Ranges of (a) calcium and (b) magnesium concentrations in leaves of
rhododendrons, grouped according to soil pH. Averages are shown by black bars. The ranges for typical woody plants are also shown. |
IRON AND MANGANESE
Magnesium in chlorophyll plays an essential role in absorbing the sun's energy for photosynthesis.
The subsequent energy transfer processes within plant cells use compounds containing iron, while
manganese is involved in the stage where more light energy is absorbed and oxygen is emitted to the
atmosphere. Both iron and manganese are readily transferred from acid soils to plants, but at high
pH they become less available, forming relatively insoluble oxides. However, the process of uptake
by plant roots, particularly for manganese, is extremely complex, and there is no simple correlation
between availability and pH.
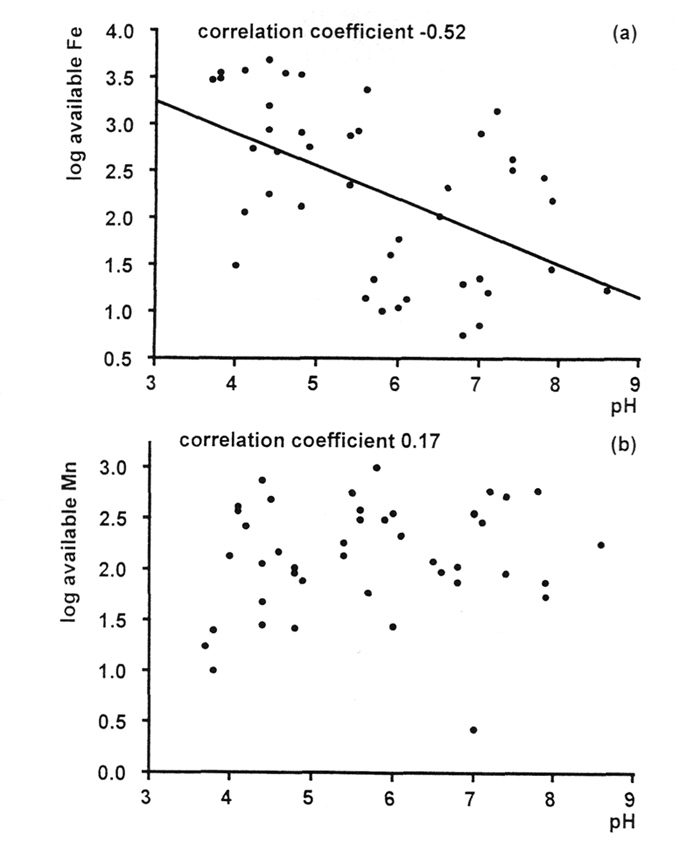
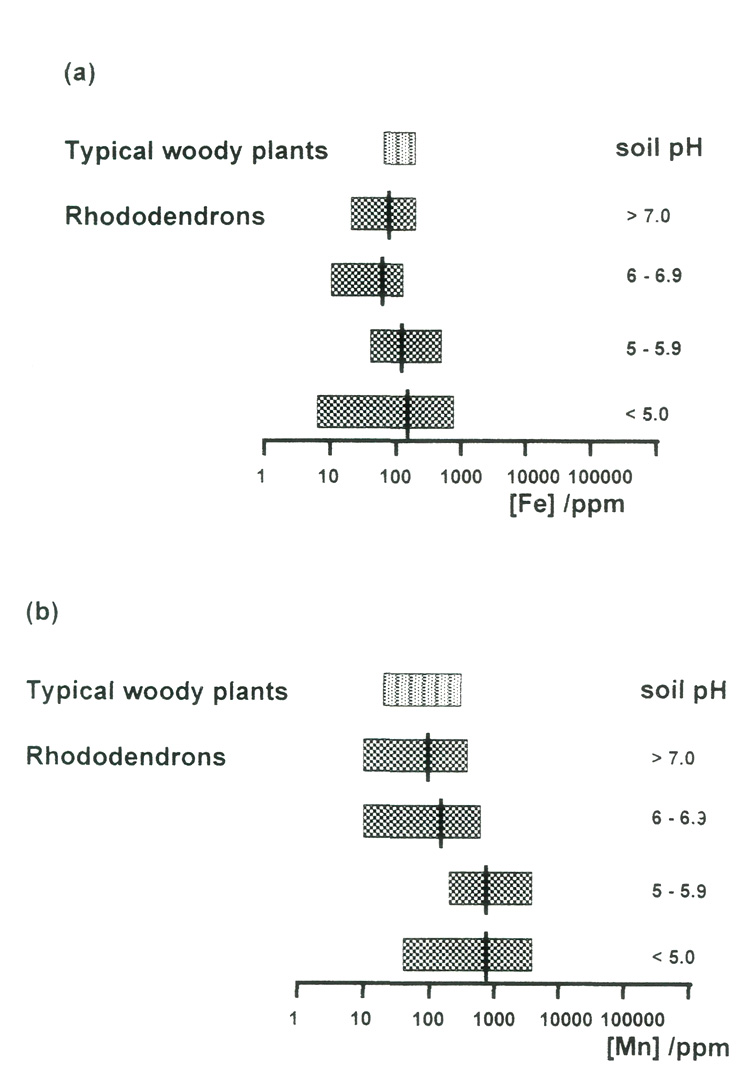
Figure 7(a) shows the correlation between available iron and pH for the samples of soils from Rhododendron roots. The correlation is not very strong, but it is clearly negative; more alkaline conditions reduce availability of iron. But if we look at the amounts of iron that are present in the leaves of the plants growing on these soils [Figure 8(a)], we can see that there are some in all pH ranges, acid and alkaline, that fall below the normal range for woody plants (Table 1). This suggests that there is a risk of iron deficiency (although it was not visibly evident in any of these specimens), but not just in the plants growing in the most alkaline conditions. Fortunately, it is relatively easy to treat iron deficiency, by administering it in sequestered form (as its EDTA complex), so this should not be an insuperable obstacle to growing rhododendrons in soils containing limestone. On the other hand, plants growing in the most alkaline conditions may be able to find more than sufficient iron for healthy growth.
| Table 1. Low iron concentrations in Rhododendron leaves.</tr> | ||||
| Species | soil pH | [Fe] / ppm | ||
| R. selense | 4.0 | 6 | ||
| R. wardii W. W. Sm. x vernicosum | 4.0 | 9 | ||
| R. phaeochrysum Balf. f. W. W. Sm. hybrid | 3.7 | 11 | ||
| R. vernicosum | 3.8/4.8/6.8 | 26/26/11 | ||
| R. uvariifolium | 6.0 | 13 | ||
| R. cuneatum | 7.9 | 20 | ||
| R. primuliflorum | 7.1 | 21 | ||
| R. decorum | 7.0 | 22 | ||
| R. aganniphum Balf. f. Kingdon Ward | 3.7 | 24 | ||
 |
|
Figure 7. Correlation pH and concentrations of (a) available iron and (b) available manganese in Rhododendron root soils. |
The leaf manganese data in Figure 8(b) do show a (negative) correlation with soil pH, but there is an enormous total range (a ratio of nearly 400 between the largest and smallest concentrations), and there are very wide ranges within each pH band. There are a few specimens for which the concentration falls below the normal range for woody plants, and a large number for which it exceeds the normal maximum by a large amount. It is instructive to consider both of these categories.
The lowest manganese leaf concentrations, all of which were found in plants growing in soils of high pH, are given in Table2. The lower limit for other plant species is reckoned to be between 15 and 30 ppm, so a few specimens are clearly right on the verge of suffering from manganese deficiency. It appears that they can just find sufficient manganese to survive, even in the extreme condition of growing on almost pure powdered limestone. Also in Table 2 are data for two Rhododendron species growing on serpentine, which has a similarly high pH, but very small amounts of calcium. The manganese data are again right on the lower acceptable limit, so it seems that manganese availability may be a critical factor.
| Table 2. Low manganese concentrations in Rhododendron leaves.</tr> | ||||
| Species | soil pH | [Mn] / ppm | ||
| (a) on limestone | ||||
| R. rupicola | 7.1 | 11 | ||
| R. aganniphum | 6.8 | 11 | ||
| R. telmateium | 7.9 | 14 | ||
| R. cuneatum | 7.9 | 24 | ||
| (b) on serpentine | ||||
| R. occidentale | 6.5 | 29 | ||
| R. neoglandulosum | 6.8 | 11 | ||
 |
|
Figure 8. Ranges of (a) iron and (b) manganese concentrations in the leaves of rhododendrons,
grouped according to soil pH. Averages are shown by black bars. The ranges for typical woody plants are also shown. The very wide range of manganese concentrations is significant. |
FACTORS AFFECTING UPTAKE OF MANGANESE
Although the amount of so-called "available" iron and manganese in soils is reduced at
high pH, this does not mean that this one factor alone can account for the rates of uptake.
The mechanisms by which they are transferred from soil to roots to leaves are complex, particularly
so in the case of manganese, and there are conflicting reports in the literature (Graham et al.,
1988). Soil bacteria which reduce manganese from its oxidized form (manganese dioxide, MnO2)
to its reduced form (complexes of Mn2+ ions) play an important role. These bacteria
cause the rate of transfer to increase with pH, so countering the effects of the reduction in available
amounts with pH. Moreover, the action of the bacteria is stimulated or their numbers are
increased by root hair exudates (Graham etal., 1988).
Other factors over which we may have more control are also significant. Water logging enhances reduction of manganese dioxide, so the heavy summer rainfall in monsoon areas may have a positive role on manganese uptake, rather than simply washing salts out of the soil. Iron deficiency can also be countered in this way. On the other hand, experimentally determined concentrations of manganese are increased if soil samples are dried, suggesting that the drying process may also release trapped ions. This raises the possibility that the combination of dry and wet seasons may be of more importance than the occurrence of either one type of weather. The areas in which we have studied wild rhododendrons are very dry in spring and extremely wet in summer. In addition, it has been reported that peat can reduce availability of manganese in soils, as the metal becomes bound tightly to some components of the peat, while clay minerals can trap manganese and other metals, again reducing their availability. These main sources, stores and sinks of manganese are summarized diagramatically in Figure 9.
How to Grow Rhododendrons on Limestone
In this work we have tried to establish facts and to avoid undue speculation, as this area
has suffered from unwarranted assumptions in the past. But the processes of plant nutrition
are complex, particularly so for manganese, and in considering these factors we have inevitably
had to be less definite than we would like. Nevertheless, we are aware that readers will want to
know how to grow rhododendrons on limestone (and other alkaline) soils. We therefore offer
some suggestions, with the warning that this section of this paper is based on our observations
of wild plants, and has not been tested. We hope that it will encourage adventurous cultivation
techniques, and that we will hear about the successes and failures - preferably more successes.
First, the species that have been observed growing in the most extreme conditions should be tried. Rhododendron telmateium and R. cuneatum are the first choices, although other members of the Lapponica sub-section may be equally amenable. Rhododendron primuliflorum has been widely reported as a lime-lover (Cox and Cox, 1997), and that is also our experience, with it usually being found in crevices in limestone rocks, as is R. rupicola [Photograph 3]; R. racemosum Franch. is more usually found in flatter, more open areas. Of the larger species, R. vernicosum Franch., R. decorum Franch. and R. yunnanense Franch. are the first choices. Members of the Taliensia sub-section are frequently found in soils with pH above 6, but not in the most extreme alkaline situations. Rhododendron occidentale and R. neoglandulosum Harmaja are the best examples of species flourishing on serpentine, and it will be interesting to learn whether they are equally at home on limestone.
Secondly, lime-tolerant strains should be sought and developed. Seed from plants growing in the most alkaline soils should be collected, and then sown in lime-rich soil. Traditionally, plants have always been raised in acid media, and so unconsciously there has been selection against lime-tolerance in cultivated plants.
Once the material with the best chance of success has been obtained, how should the plants be cultivated? Again, traditional methods may not be the best. Normally one would add peat, both to provide acid conditions and to retain moisture; an acidifier (perhaps sulfur) could be added to the soil; the soil would be kept damp throughout the year; and chlorosis would be tackled by addition of sequestered iron, which usually contains a proportion of manganese as well.
However, as we have seen, peat can decrease the availability of essential minerals, as it holds tightly on to some metal ions. Acidifiers may be of help, but in soils which contain significant amounts of limestone the continuing dissolution of the rock will soon overcome the added acidifier. Continuous dampness may well also be counter-productive, as manganese availability is increased during a dry period, although a period of water logging can then help the uptake of available manganese by plants. Addition of sequestered iron is certainly helpful if iron deficiency is the primary cause of chlorosis, but there is little evidence that this is the primary problem with rhododendrons on lime-rich soils. And the manganese which comes with the commercial sequestered iron is not much help at all. The manganese EDTA complex which is present in these mixtures is a weak complex, which dissociates rapidly in the soil, giving the uncomplexed Mn2+ ion. This in turn is very quickly oxidized to manganese dioxide, which is unavailable to plant roots. The EDTA thus released may increase the availability of other metals, and in some cases may make manganese deficiency even worse.
What is needed is something which will provide a steady source of available manganese in the soil. How is this done in the wild? The limestone soils have much higher available manganese levels than can possibly arise from the inorganic component, derived from the rock. But rhododendrons, and other ericaceous plants, can accumulate large amounts of manganese, gathered whenever it is available. Composted ericaceous plants, preferably but not necessarily brought from a non-limestone area, may provide precisely what is needed (Figure 9).
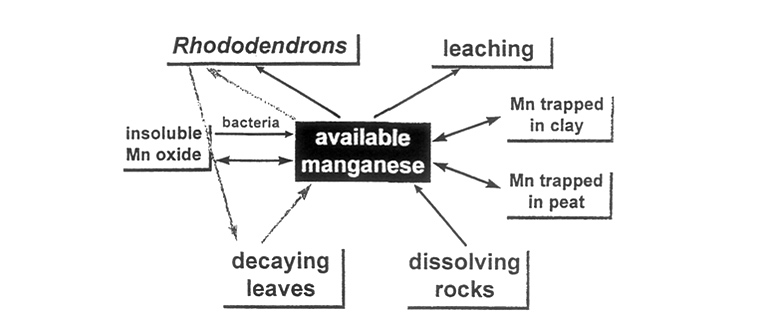 |
| Figure 9. Sources, stores and sinks of manganese in the soil. |
Future Work
Of course, there is more work to be done:
- experiments growing rhododendrons in lime-rich soil;
- study of other plants, particularly other members of the Ericaceae, but also other genera, to see whether the behavior of rhododendrons is typical of so-called "calcifuges";
- study of plants which appear to be suffering from growing on limestone or other alkaline soils;
- following the uptake of essential minerals through the year;
- comparing the effects of limestone and serpentine.
Experimental Methods
The pH of soil samples was measured in the field using a portable pH meter (Hanna Instruments pHep,
with temperature compensation) and in the laboratory on collected samples. Where readings were
made for the same samples by the two methods the results were satisfactorily consistent.
Soil samples were collected from the region through which roots of the plants were growing, usually in the top 10-20 cm, ignoring any surface litter. Samples were also taken from any distinct soil zones which were accessible at lower levels. Soils were dried in air before being sealed for transport. Wherever possible, specimens of rock within or below the soils were also collected. The amount of rock in the soil, particularly within the root ball, was noted. Leaves were collected from the same individual plants whose root-soil was sampled, and were pressed and dried. Table 3 lists all the species studied, with observed soil pH, grouped in the ranges up to 4.9, 5.0 - 5.9, 6.0 - 6.9 and 7.0 upwards.
| Table 3. Species of Rhododendron studied. | ||||
| Species | pH | |||
| up to 4.9 | 5.0-5.9 | 6.0-6.9 | 7.0 up | |
| R. adenogynum | * | * | ||
| R. aganniphum x phaeochrysum | * | |||
| R. aganniphum | * | * | * | |
| R. beesianum | * | |||
| R. clementinae x R. phaeochrysum | * | |||
| R. clementinae | * | * | ||
| R. cuneatum | * | |||
| R. decorum | * | |||
| R. hippophaeoides | * | * | ||
| R. microphyton | * | |||
| R. oreotrephes | * | * | ||
| R. phaeochrysum | * | * | ||
| R. primuliflorum | * | * | ||
| R. racemosum | * | |||
| R. roxieanum hybrid | * | |||
| R. rubiginosum | * | * | * | |
| R. rupicola ssp. chryseum | * | * | ||
| R. rupicola | * | * | ||
| R. selense | * | * | ||
| R. telmateium | * | |||
| R. uvariifolium | * | * | * | |
| R. vernicosum | * | * | * | |
| R. wardii | * | |||
| R. yungningense | * | * | ||
| R. yunnanense | * | * | ||
The organic content of soil samples was measured by combustion. Concentrations of the four key metals, calcium, magnesium, iron and manganese, as well as phosphorus, were measured by Inductively-Coupled Plasma Atomic Emission Spectroscopy for solutions extracted with (a) deionized water, (b) ammonium acetate / acetic acid solution at pH 4 and (c) hydroxylamine / nitric acid solution at pH 2. The first of these corresponds to rain water in contact with the soil for 16 hours, which depending on weather may be longer or shorter than happens naturally. Both rain and deionized water will be saturated with atmospheric carbon dioxide, and thus have a pH of about 5.6. Extract (b) corresponds to the amounts of metals ions available to the plants, and (c) to the total amounts present in the soil. In some cases aluminum, potassium, copper, zinc, chromium and nickel concentrations were also measured. Concentrations of metal ions in the water samples were also measured. Rocks were analyzed by X-ray Fluorescence in the Department of Geology, University of Edinburgh.
Leaves were dried and weighed, and then treated with concentrated nitric acid, which gave a solution containing the dissolved metal ions. After filtration the solutions were analyzed for calcium, magnesium, iron, manganese, phosphorus (and in some cases potassium, copper and zinc) in the same way as the soil extract solutions.
Acknowledgements
We thank the Alpine Garden Society, the American Rhododendron Society, the Davis Fund of
the University of Edinburgh, the Merlin Trust, The Royal Society, The Royal Society of
Edinburgh, and the Scottish Rock Garden Club for financial support, and Dr. David Chamberlain
(Royal Botanic Garden, Edinburgh), Guan Kaiyun, Sun Hang and Cheng Xiao (Kunming Institute
of Botany), Dr. David Millward (British Geological Survey), Dr. David White, Alison Corteen
and Cheryl Wiramanaden (University of Edinburgh) and Andrew Rankin (University of Southampton)
for assistance. Soils were imported into the UK under license IMP/SOIL/21/1997.
Literature cited
Chaanin, A. 1998. Lime tolerance in rhododendron. Comb. Proc. IPPS 48: 180-182.
Chaanin, A.; Preil, W. 1994. Acta Horticulturae364: 71-77.
Chaanin, A.; Preil, W. 1996. Limits for bicarbonate concentrations in rhododendron
rootstocks. TASPO-Garten-baummagazin 5(2): 44-46.
Cox, E. H. M. 1945. London: The Scientific Book Guild, pp. 29, 162.
Cox, P. A.; Cox, K. N. E. 1997. The Encyclopaedia of Rhododendron Species.
Glencarse, Perth: Glendoick Publishing.
Drehmel, G.; Preil, W. 1992. Rhododendron und immergrne Laubgeh lzejahrbuch 23.
Bremen, Gemany: Deutsche Rhododendron Gesellschaft.
Graham, R. D.; Hannam, R. J.; Uren, N. C. 1988. Manganese in Soils and Plants.
Dordrecht, Boston and London: KluwerAcademic Publishers.
Grove, A. 1927. Rhododendrons and lime. Gardeners' Chronicle 82:426-428.
Hartge, K. H. 1956. Rhododendron und immergrne Laubgehlzejahrbuch 27. Bremen, Germany:
Deutsche Rhododendron Gesellschaft.
Kingdon Ward, F.1926. Rhododendrons for everyone. London: The Gadeners' Chronicle Ltd.
Korcak, R. F. 1988. Horticultural Reviews. 10: 183.
Leiser, A. T. 1957. Rhododendron and Camellia Year Book 11: 47-51.
McAleese, A. J.; Rankin, D. W. H.; Sun, H. 1999. The New Plantsman 6: 23-29.
Tod, H. 1956. J. Scot. Rock Gard. Club 5: 50-56.
Tod, H. 1971a. Gardeners' Chronicle/HTJ 170, issue22: 17-18
Tod, H. 1971b. Gardeners' Chronicle/HTJ 1 70, issue 23: 22-25.
Widrlechner, M. P.; Larson, R. A.; Dragula, S. K. 1993. Exploring the deciduous azaleas
and elepidote rhododendrons of the Midwestern United States. J. Am. Rhodo. Soc. 47(3): 153-156.
* A high pH value (over 7) indicates alkalinity and a low value indicates acidity. The soils studied thus ranged from quite strongly acidic to mildly alkaline. Highly alkaline soils are very rare, and can not be caused by limestone.
</div> by Chase Dooley
 |
 |
|
Photograph 1. The limestone mountains of Western China: the Yulong Shan in North-West Yunnan Province. |
Photograph 2. Rhododendron telmateium growing in limestone. The soil has been cut away to reveal the root zone. |
Commonly Held Theories of How Rhododendrons Grow on Limestone
Rhododendrons growing over limestone are in fact rooted in pockets or layers of organic material,
out of contact with the limestone (Grove, 1927). Figure 1 shows the percentage of organic content
in the soils, and indicates that few soils are predominantly organic, and that most soils are less
than 50% organic, regardless of their acidity or alkalinity. Moreover, analyses for calcium, which
is the metal that is the predominant component of limestone (calcium carbonate), show that its
concentration correlates strongly with pH [Figure 2(a)].
These data confirm what is obvious by inspection; there are some Rhododendron plants which quite clearly have their roots in white, powdered limestone, with very little organic material present at all [photograph 2]. Of course, there are plenty of places where there is a thick layer of organic-rich material over the limestone, but this does not mean that such a layer is ubiquitous. We therefore reach by analysis the same conclusion that George Forrest reached in 1915, using only his eyes: "Most of the rhododendrons in that region (North-West Yunnan) grow directly in, or on, pure limestone" (Cox, 1945).
 |
|
Figure 1. Organic content of soils as a function of pH. Averages are shown by shaded bars,
individual measurements by black bars. Most soils in all pH ranges have a high non-organic content, i.e., of material derived from rock. |
Companion calciphile plants also confirm that some rhododendrons are happily adapted to very alkaline conditions. For example, Rhododendron telmateium Balf. f. W. W. Sm. and R. cuneatum W. W. Sm. grow alongside such confirmed lime-lovers as Primula forrestii and Daphne calcicola, while we have found R. primuliflorum Bureau Franch. and R. rupicola W. W. Sm. growing in limestone crevices shared with Paraquilegia anemonoides, another species associated uniquely with limestone.
The limestone in which the rhododendrons grow is dolomitic (Hartge, 1956). This could be highly significant. Dolomite is a mixture of equal amounts of calcium carbonate and magnesium carbonate. Calcium is a bit heavier than magnesium, so equal proportions gives 45% magnesium carbonate by weight.
 |
|
Photograph 3. Rhododendron rupicola growing in limestone crevices. |
Dolomitic limestone is the term used for rock which has upwards of 2.5% magnesium carbonate. Magnesium is an essential part of chlorophyll, the green pigment in leaves which absorbs energy from sunlight and allows growth. The theory is that calcium, which is chemically similar, competes with the magnesium for uptake by the roots of the plants, and so hinders growth. However, our analyses show that the samples of limestone from Yunnan only have between 0.2 and 1.6% magnesium carbonate, so they are certainly not dolomitic (Figure 3). And as we shall see, a large excess of calcium does not inhibit take-up of magnesium anyway. The amount of available magnesium in our soil samples was generally about one-tenth of that of calcium, and increased with pH ([Figure 2(b)] - it certainly did not become less available in alkaline soils.
 |
|
Figure 2. Correlation of pH and concentrations of (a) available calcium and (b) available magnesium in Rhododendron root soils. |
The limestone is hard and more or less insoluble (Tod, 1971 a, 1971 b). Our geologist colleagues have labeled all the limestone specimens as soft, and that is consistent with their easy erosion, and particularly with their dispersion as fine particles in glacier melt-water. We have analyzed soils for what is known as "available" calcium, i.e., the part in the soil which is sufficiently soluble to be available for uptake by the plant roots. The data shown in Figure 2 confirm that the calcium carbonate is not locked up in hard, insoluble rock.
 |
|
Figure 3. Composition of dolomite and dolomitic limestones. The amount of magnesium
in limestone samples from North-West Yunnan is far too low for them to be classified as dolomitic. |
Persistent heavy rain in these monsoon-affected areas washes any dissolved limestone out of the soil (Hartge, 1956). There are three difficulties with this. First, as we have seen, the calcium is clearly present in the soil samples. Secondly, the idea of washing the dissolved material out of the soil is only possible if there is an organic layer above the limestone rock, and separate from it. We have shown that the organic layer is mixed with, and sometimes dominated by, the inorganic (rock-derived) component. Thirdly, the rain is not incessant; spring and fall are dry seasons, when moisture at the roots of rhododendrons comes primarily by capillary action from below, or may move slowly downwards after any rain. Either way, salts dissolved from the underlying rocks can diffuse upwards into the root zone (Figure 4).
 |
|
Figure 4. When a lot of water flows through peaty soil, metal salts are slowly
washed through (leached), although they are held quite tightly by the peat. When the water flow is low, diffusion of metal ions can be sufficient to transport salts upwards, against the flow of water. |
We cannot escape the conclusion that there are situations in which rhododendrons do grow with their roots in contact with available limestone, and that this does them no harm. So we have had to reject all of these theories. Perhaps we should not be surprised: there are plenty of other ericaceous plants which grow in alkaline soils. But we are left with the question, "Why do most rhododendrons in cultivation dislike limestone?" It cannot be that they are short of magnesium, nor that calcium in the soil is toxic to them. The latter has also been shown by careful cultivation experiments (Drehmel and Preil, 1992), although Tod (1956) assumed calcium toxicity to be significant, solely on the basis of an experiment involving soil with high pH induced by addition of magnesium carbonate. Nor can it be caused by the carbonate ions from the limestone (at high pH) or bicarbonate (at somewhat lower pH), because they are abundant in our soil samples.
Bicarbonate ion has been claimed to be significantly toxic to rhododendrons (Chaanin and Preil, 1994, 1996; Chaanin, 1998), but it should be noted that merely adding bicarbonate solutions to soil does not necessarily increase its concentration in the soil, because it is in equilibrium with atmospheric carbon dioxide; conversely, it will always be present in soil in the appropriate pH range (around pH 6), whether or not it has been specifically added as bicarbonate.
If all these more straightforward explanations of rhododendrons appearing to tolerate high pH soils in some wild sites fail, we must turn our attention to the foliage of the plants, and to iron and manganese, two other metals which are essential for plant growth and which must be obtained from the soil. However, we also need to recognize that concentrations of the relevant chemical species in solution are interdependent (Figure 5). Changing the concentration of any one constituent changes all the others as well, and this diagram shows only a tiny fraction of all the components that are involved. Moreover, concentrations in aqueous solution in the soil are not the same as those in or on the surface of soil solids. Organic and inorganic materials interact in different ways with different ions, so that the whole system is extremely complicated.
 |
|
Figure 5. Concentrations of compounds and ions important to plants are linked by a
series of inter-related equilibria. Concentrations within organic and non-organic solids in the soil will be quite different from those in aqueous solution. |
Results and Discussion
CALCIUM AND MAGNESIUM IN THE FOLIAGE
Measurements of calcium concentration in the leaves of rhododendrons confirm their remarkable
tolerance of a huge range of calcium concentrations in the soil. Figure 2(a) showed that
there was a factor of 1000 between the highest and lowest available amounts of calcium in the
sample soils, and that this concentration correlated strongly with soil pH. Figure 6(a) displays
the concentration ranges of calcium in the leaves of rhododendrons, grouped according to the
pH of the soils in which they were growing, and shows that there is no correlation with pH at all.
There is a ratio of only 15 between the highest and lowest, for 80 leaves from 80 separate plants
on 40 sites, so uptake of calcium is clearly well controlled, and the insensitivity of plants to
available soil calcium is demonstrated. Much the same applies to magnesium. The soil concentrations
[Figure 2(b)] were about a factor of 10 lower than those of calcium, and the same is true for the
leaf concentrations [Figure 6(b)]. In both cases the observed ranges for rhododendrons are somewhat
lower than is typical for woody plants, as has been noted previously (Tod, 1971b).
 |
|
Figure 6. Ranges of (a) calcium and (b) magnesium concentrations in leaves of
rhododendrons, grouped according to soil pH. Averages are shown by black bars. The ranges for typical woody plants are also shown. |
IRON AND MANGANESE
Magnesium in chlorophyll plays an essential role in absorbing the sun's energy for photosynthesis.
The subsequent energy transfer processes within plant cells use compounds containing iron, while
manganese is involved in the stage where more light energy is absorbed and oxygen is emitted to the
atmosphere. Both iron and manganese are readily transferred from acid soils to plants, but at high
pH they become less available, forming relatively insoluble oxides. However, the process of uptake
by plant roots, particularly for manganese, is extremely complex, and there is no simple correlation
between availability and pH.
Figure 7(a) shows the correlation between available iron and pH for the samples of soils from Rhododendron roots. The correlation is not very strong, but it is clearly negative; more alkaline conditions reduce availability of iron. But if we look at the amounts of iron that are present in the leaves of the plants growing on these soils [Figure 8(a)], we can see that there are some in all pH ranges, acid and alkaline, that fall below the normal range for woody plants (Table 1). This suggests that there is a risk of iron deficiency (although it was not visibly evident in any of these specimens), but not just in the plants growing in the most alkaline conditions. Fortunately, it is relatively easy to treat iron deficiency, by administering it in sequestered form (as its EDTA complex), so this should not be an insuperable obstacle to growing rhododendrons in soils containing limestone. On the other hand, plants growing in the most alkaline conditions may be able to find more than sufficient iron for healthy growth.
| Table 1. Low iron concentrations in Rhododendron leaves.</tr> | ||||
| Species | soil pH | [Fe] / ppm | ||
| R. selense | 4.0 | 6 | ||
| R. wardii W. W. Sm. x vernicosum | 4.0 | 9 | ||
| R. phaeochrysum Balf. f. W. W. Sm. hybrid | 3.7 | 11 | ||
| R. vernicosum | 3.8/4.8/6.8 | 26/26/11 | ||
| R. uvariifolium | 6.0 | 13 | ||
| R. cuneatum | 7.9 | 20 | ||
| R. primuliflorum | 7.1 | 21 | ||
| R. decorum | 7.0 | 22 | ||
| R. aganniphum Balf. f. Kingdon Ward | 3.7 | 24 | ||
 |
|
Figure 7. Correlation pH and concentrations of (a) available iron and (b) available manganese in Rhododendron root soils. |
The leaf manganese data in Figure 8(b) do show a (negative) correlation with soil pH, but there is an enormous total range (a ratio of nearly 400 between the largest and smallest concentrations), and there are very wide ranges within each pH band. There are a few specimens for which the concentration falls below the normal range for woody plants, and a large number for which it exceeds the normal maximum by a large amount. It is instructive to consider both of these categories.
The lowest manganese leaf concentrations, all of which were found in plants growing in soils of high pH, are given in Table2. The lower limit for other plant species is reckoned to be between 15 and 30 ppm, so a few specimens are clearly right on the verge of suffering from manganese deficiency. It appears that they can just find sufficient manganese to survive, even in the extreme condition of growing on almost pure powdered limestone. Also in Table 2 are data for two Rhododendron species growing on serpentine, which has a similarly high pH, but very small amounts of calcium. The manganese data are again right on the lower acceptable limit, so it seems that manganese availability may be a critical factor.
| Table 2. Low manganese concentrations in Rhododendron leaves.</tr> | ||||
| Species | soil pH | [Mn] / ppm | ||
| (a) on limestone | ||||
| R. rupicola | 7.1 | 11 | ||
| R. aganniphum | 6.8 | 11 | ||
| R. telmateium | 7.9 | 14 | ||
| R. cuneatum | 7.9 | 24 | ||
| (b) on serpentine | ||||
| R. occidentale | 6.5 | 29 | ||
| R. neoglandulosum | 6.8 | 11 | ||
 |
|
Figure 8. Ranges of (a) iron and (b) manganese concentrations in the leaves of rhododendrons,
grouped according to soil pH. Averages are shown by black bars. The ranges for typical woody plants are also shown. The very wide range of manganese concentrations is significant. |
FACTORS AFFECTING UPTAKE OF MANGANESE
Although the amount of so-called "available" iron and manganese in soils is reduced at
high pH, this does not mean that this one factor alone can account for the rates of uptake.
The mechanisms by which they are transferred from soil to roots to leaves are complex, particularly
so in the case of manganese, and there are conflicting reports in the literature (Graham et al.,
1988). Soil bacteria which reduce manganese from its oxidized form (manganese dioxide, MnO2)
to its reduced form (complexes of Mn2+ ions) play an important role. These bacteria
cause the rate of transfer to increase with pH, so countering the effects of the reduction in available
amounts with pH. Moreover, the action of the bacteria is stimulated or their numbers are
increased by root hair exudates (Graham etal., 1988).
Other factors over which we may have more control are also significant. Water logging enhances reduction of manganese dioxide, so the heavy summer rainfall in monsoon areas may have a positive role on manganese uptake, rather than simply washing salts out of the soil. Iron deficiency can also be countered in this way. On the other hand, experimentally determined concentrations of manganese are increased if soil samples are dried, suggesting that the drying process may also release trapped ions. This raises the possibility that the combination of dry and wet seasons may be of more importance than the occurrence of either one type of weather. The areas in which we have studied wild rhododendrons are very dry in spring and extremely wet in summer. In addition, it has been reported that peat can reduce availability of manganese in soils, as the metal becomes bound tightly to some components of the peat, while clay minerals can trap manganese and other metals, again reducing their availability. These main sources, stores and sinks of manganese are summarized diagramatically in Figure 9.
How to Grow Rhododendrons on Limestone
In this work we have tried to establish facts and to avoid undue speculation, as this area
has suffered from unwarranted assumptions in the past. But the processes of plant nutrition
are complex, particularly so for manganese, and in considering these factors we have inevitably
had to be less definite than we would like. Nevertheless, we are aware that readers will want to
know how to grow rhododendrons on limestone (and other alkaline) soils. We therefore offer
some suggestions, with the warning that this section of this paper is based on our observations
of wild plants, and has not been tested. We hope that it will encourage adventurous cultivation
techniques, and that we will hear about the successes and failures - preferably more successes.
First, the species that have been observed growing in the most extreme conditions should be tried. Rhododendron telmateium and R. cuneatum are the first choices, although other members of the Lapponica sub-section may be equally amenable. Rhododendron primuliflorum has been widely reported as a lime-lover (Cox and Cox, 1997), and that is also our experience, with it usually being found in crevices in limestone rocks, as is R. rupicola [Photograph 3]; R. racemosum Franch. is more usually found in flatter, more open areas. Of the larger species, R. vernicosum Franch., R. decorum Franch. and R. yunnanense Franch. are the first choices. Members of the Taliensia sub-section are frequently found in soils with pH above 6, but not in the most extreme alkaline situations. Rhododendron occidentale and R. neoglandulosum Harmaja are the best examples of species flourishing on serpentine, and it will be interesting to learn whether they are equally at home on limestone.
Secondly, lime-tolerant strains should be sought and developed. Seed from plants growing in the most alkaline soils should be collected, and then sown in lime-rich soil. Traditionally, plants have always been raised in acid media, and so unconsciously there has been selection against lime-tolerance in cultivated plants.
Once the material with the best chance of success has been obtained, how should the plants be cultivated? Again, traditional methods may not be the best. Normally one would add peat, both to provide acid conditions and to retain moisture; an acidifier (perhaps sulfur) could be added to the soil; the soil would be kept damp throughout the year; and chlorosis would be tackled by addition of sequestered iron, which usually contains a proportion of manganese as well.
However, as we have seen, peat can decrease the availability of essential minerals, as it holds tightly on to some metal ions. Acidifiers may be of help, but in soils which contain significant amounts of limestone the continuing dissolution of the rock will soon overcome the added acidifier. Continuous dampness may well also be counter-productive, as manganese availability is increased during a dry period, although a period of water logging can then help the uptake of available manganese by plants. Addition of sequestered iron is certainly helpful if iron deficiency is the primary cause of chlorosis, but there is little evidence that this is the primary problem with rhododendrons on lime-rich soils. And the manganese which comes with the commercial sequestered iron is not much help at all. The manganese EDTA complex which is present in these mixtures is a weak complex, which dissociates rapidly in the soil, giving the uncomplexed Mn2+ ion. This in turn is very quickly oxidized to manganese dioxide, which is unavailable to plant roots. The EDTA thus released may increase the availability of other metals, and in some cases may make manganese deficiency even worse.
What is needed is something which will provide a steady source of available manganese in the soil. How is this done in the wild? The limestone soils have much higher available manganese levels than can possibly arise from the inorganic component, derived from the rock. But rhododendrons, and other ericaceous plants, can accumulate large amounts of manganese, gathered whenever it is available. Composted ericaceous plants, preferably but not necessarily brought from a non-limestone area, may provide precisely what is needed (Figure 9).
 |
| Figure 9. Sources, stores and sinks of manganese in the soil. |
Future Work
Of course, there is more work to be done:
- experiments growing rhododendrons in lime-rich soil;
- study of other plants, particularly other members of the Ericaceae, but also other genera, to see whether the behavior of rhododendrons is typical of so-called "calcifuges";
- study of plants which appear to be suffering from growing on limestone or other alkaline soils;
- following the uptake of essential minerals through the year;
- comparing the effects of limestone and serpentine.
Experimental Methods
The pH of soil samples was measured in the field using a portable pH meter (Hanna Instruments pHep,
with temperature compensation) and in the laboratory on collected samples. Where readings were
made for the same samples by the two methods the results were satisfactorily consistent.
Soil samples were collected from the region through which roots of the plants were growing, usually in the top 10-20 cm, ignoring any surface litter. Samples were also taken from any distinct soil zones which were accessible at lower levels. Soils were dried in air before being sealed for transport. Wherever possible, specimens of rock within or below the soils were also collected. The amount of rock in the soil, particularly within the root ball, was noted. Leaves were collected from the same individual plants whose root-soil was sampled, and were pressed and dried. Table 3 lists all the species studied, with observed soil pH, grouped in the ranges up to 4.9, 5.0 - 5.9, 6.0 - 6.9 and 7.0 upwards.
| Table 3. Species of Rhododendron studied. | ||||
| Species | pH | |||
| up to 4.9 | 5.0-5.9 | 6.0-6.9 | 7.0 up | |
| R. adenogynum | * | * | ||
| R. aganniphum x phaeochrysum | * | |||
| R. aganniphum | * | * | * | |
| R. beesianum | * | |||
| R. clementinae x R. phaeochrysum | * | |||
| R. clementinae | * | * | ||
| R. cuneatum | * | |||
| R. decorum | * | |||
| R. hippophaeoides | * | * | ||
| R. microphyton | * | |||
| R. oreotrephes | * | * | ||
| R. phaeochrysum | * | * | ||
| R. primuliflorum | * | * | ||
| R. racemosum | * | |||
| R. roxieanum hybrid | * | |||
| R. rubiginosum | * | * | * | |
| R. rupicola ssp. chryseum | * | * | ||
| R. rupicola | * | * | ||
| R. selense | * | * | ||
| R. telmateium | * | |||
| R. uvariifolium | * | * | * | |
| R. vernicosum | * | * | * | |
| R. wardii | * | |||
| R. yungningense | * | * | ||
| R. yunnanense | * | * | ||
The organic content of soil samples was measured by combustion. Concentrations of the four key metals, calcium, magnesium, iron and manganese, as well as phosphorus, were measured by Inductively-Coupled Plasma Atomic Emission Spectroscopy for solutions extracted with (a) deionized water, (b) ammonium acetate / acetic acid solution at pH 4 and (c) hydroxylamine / nitric acid solution at pH 2. The first of these corresponds to rain water in contact with the soil for 16 hours, which depending on weather may be longer or shorter than happens naturally. Both rain and deionized water will be saturated with atmospheric carbon dioxide, and thus have a pH of about 5.6. Extract (b) corresponds to the amounts of metals ions available to the plants, and (c) to the total amounts present in the soil. In some cases aluminum, potassium, copper, zinc, chromium and nickel concentrations were also measured. Concentrations of metal ions in the water samples were also measured. Rocks were analyzed by X-ray Fluorescence in the Department of Geology, University of Edinburgh.
Leaves were dried and weighed, and then treated with concentrated nitric acid, which gave a solution containing the dissolved metal ions. After filtration the solutions were analyzed for calcium, magnesium, iron, manganese, phosphorus (and in some cases potassium, copper and zinc) in the same way as the soil extract solutions.
Acknowledgements
We thank the Alpine Garden Society, the American Rhododendron Society, the Davis Fund of
the University of Edinburgh, the Merlin Trust, The Royal Society, The Royal Society of
Edinburgh, and the Scottish Rock Garden Club for financial support, and Dr. David Chamberlain
(Royal Botanic Garden, Edinburgh), Guan Kaiyun, Sun Hang and Cheng Xiao (Kunming Institute
of Botany), Dr. David Millward (British Geological Survey), Dr. David White, Alison Corteen
and Cheryl Wiramanaden (University of Edinburgh) and Andrew Rankin (University of Southampton)
for assistance. Soils were imported into the UK under license IMP/SOIL/21/1997.
Literature cited
Chaanin, A. 1998. Lime tolerance in rhododendron. Comb. Proc. IPPS 48: 180-182.
Chaanin, A.; Preil, W. 1994. Acta Horticulturae364: 71-77.
Chaanin, A.; Preil, W. 1996. Limits for bicarbonate concentrations in rhododendron
rootstocks. TASPO-Garten-baummagazin 5(2): 44-46.
Cox, E. H. M. 1945. London: The Scientific Book Guild, pp. 29, 162.
Cox, P. A.; Cox, K. N. E. 1997. The Encyclopaedia of Rhododendron Species.
Glencarse, Perth: Glendoick Publishing.
Drehmel, G.; Preil, W. 1992. Rhododendron und immergrne Laubgeh lzejahrbuch 23.
Bremen, Gemany: Deutsche Rhododendron Gesellschaft.
Graham, R. D.; Hannam, R. J.; Uren, N. C. 1988. Manganese in Soils and Plants.
Dordrecht, Boston and London: KluwerAcademic Publishers.
Grove, A. 1927. Rhododendrons and lime. Gardeners' Chronicle 82:426-428.
Hartge, K. H. 1956. Rhododendron und immergrne Laubgehlzejahrbuch 27. Bremen, Germany:
Deutsche Rhododendron Gesellschaft.
Kingdon Ward, F.1926. Rhododendrons for everyone. London: The Gadeners' Chronicle Ltd.
Korcak, R. F. 1988. Horticultural Reviews. 10: 183.
Leiser, A. T. 1957. Rhododendron and Camellia Year Book 11: 47-51.
McAleese, A. J.; Rankin, D. W. H.; Sun, H. 1999. The New Plantsman 6: 23-29.
Tod, H. 1956. J. Scot. Rock Gard. Club 5: 50-56.
Tod, H. 1971a. Gardeners' Chronicle/HTJ 170, issue22: 17-18
Tod, H. 1971b. Gardeners' Chronicle/HTJ 1 70, issue 23: 22-25.
Widrlechner, M. P.; Larson, R. A.; Dragula, S. K. 1993. Exploring the deciduous azaleas
and elepidote rhododendrons of the Midwestern United States. J. Am. Rhodo. Soc. 47(3): 153-156.
* A high pH value (over 7) indicates alkalinity and a low value indicates acidity. The soils studied thus ranged from quite strongly acidic to mildly alkaline. Highly alkaline soils are very rare, and can not be caused by limestone.
</div> by Chase Dooley