QBARS - v27n4 Yellow Flower Pigments in Rhododendron: A Review for Breeders
Yellow Flower Pigments in Rhododendron: A Review for Breeders
Frank S. Santamour Jr., and Robert L. Pryor
Research Geneticist and Research Horticulturist, respectively
U. S. National Arboretum, Agricultural Research Service
U. S. Department of Agriculture, Washington, D.C.
FLORAL PIGMENTS
There are two main classes of flower petal pigments in the genus
Rhododendron
: the anthocyanins and the carotenoids.
The anthocyanins belong to the flavonoid group of pigments and are responsible for the majority of pink, red, violet, and blue colors in the flowers of higher plants. These pigments are water soluble and occur in the cell sap. Anthocyanins normally occur in plants as glycosides (with sugars attached); as in cyanidin 3-galactoside, (I), Fig. 44, a major anthocyanin pigment in some red-flowered Rhododendron species.
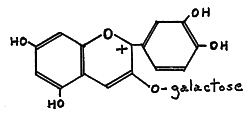
|
| FIG. 44. (I) Cyanidin 3-galactoside. |
The carotenoid class of compounds includes yellow, orange, and red pigments. These pigments are soluble in hydrocarbon solvents (carotenes) or ethyl alcohol (xanthophylls) and occur in specialized protoplasmic bodies (the plastids) in higher plants. Carotenoids are present, with the chlorophylls, in the chloroplasts of green leaves and immature fruit. As leaves approach senescence and fruits mature, the chloroplasts "degenerate" into chromoplasts. With the breakdown of the photosynthetic apparatus, the production of carotenoids increases. Carotenoids also occur in chromoplasts of non-photosynthetic flowers and roots.
Most carotenoids, such as lutein (II), Fig. 45, are yellow to orange. Red carotenoids are known as major pigments in tomato fruit and yew (Taxus) berries.
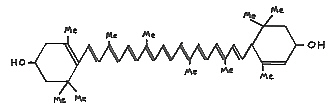
|
|
FIG. 45. (II) Lutein - a common carotenoid in yellow
flower petals. Me = methyl (CH3) group. |
Whereas the anthocyanin glycosides have closely related structures and normally occur alone or in mixtures of only a few compounds, the carotenoids have widely variable structures and mixtures of 10 or more compounds in the same organ are not uncommon. Even within a genus, the major petal carotenoids may vary considerably in different species (e.g., Mimulus-monkey-flower).
With all of this explanatory material on carotenoids, the reader might now expect some good hard data on the kind and distribution of carotenoids in yellow Rhododendron flowers. The fact is that the carotenoids of Rhododendron have not yet been positively identified. The assumption, a valid one we think, that carotenoids are present and are important pigments in yellow petals of Rhododendron has been made because the majority of the yellow pigments do not belong to any other class. We hope that research to determine the petal carotenoids in Rhododendron will be undertaken in the next few years.
The discovery that there was a class of non-carotenoid yellow pigments in Rhododendron was made rather recently, and a detailed chronology of published information is of interest. Harborne (1962) found an unusual yellow pigment in R. campylocarpum Hook.f. In later work, Harborne (1965) identified this yellow pigment as the rare flavonol quercetagetin 3-galactoside and noted its presence as a major floral pigment in R. chryseum Balf. f. & Ward; R. herpesticum Balf. f. & Ward; R. telopeum Balf. f. & Forrest; R. trichocladum Franch; and R. wardii W. W. Smith. Species and cultivars having yellow flowers and not containing quercetagetin included R. ambiguum Hemsl.; R. wightii Hook. f.; and R. cinnabarinum Hook. f. 'Lady Chamberlain'.
Re-investigation of these pigments (Harborne, 1969) showed that they were not galactosides of quercetagetin, but rather of gossypetin (III), Fig. 46. From a breeder's point of view, the distinction my be of only academic interest because both flavonols produce approximately the some colors in flowers and both are derived from hydroxylation of quersetin.
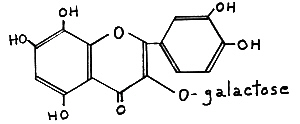
|
|
FIG. 46. (III) Gossypetin 3-galactoside -
|
A recent survey (Harborne and Williams, 1971) of 206 Rhododendron species, varieties, and cultivars showed that gossypetin was present in the leaves of 76 percent of the taxa. This study also revealed that gossypetin was the yellow flower pigment in R. ambiguum Hemsl. (earlier noted as lacking gossypetin; R. adenophorum Balf. f. W.W. Sm.; and R. anthosphaerum Diels; and in the purple-pink flowers of R. ponticum L., where it is masked by anthocyanins. Thus, although gossypetin is a common leaf constituent, it is relatively rare in flowers.
Among the azaleas (subgenus Anthodendron), the evergreen species of Sect. Tsutsutsi contain gossypetin in their leaves but the deciduous species of Sect. Pentanthera lack this flavonol. No azalea was found that produced gossypetin in the flower petals.
| Table 1. Yellow flower pigments in Rhododendron species. | |
| FLAVONOL (Gossypetin) | CAROTENOID |
| Elepidote | Elepidote |
| R. adenophorum | R. caloxanthum |
| R. anthosphaerum | R. dichroanthum |
| R. campylocarpum | (=scyphocalyx) |
| R. herpesticum | R. lacteum |
| R. telopeum | R. wightii |
| R. wardii | |
| Lepidote | R. cinnabarinum |
| R. ambiguum1 | R. keiskei |
| R. chryseum | R. lutescens |
| R. trichocladum | R. sargentianum |
| Azaleas | |
| R. austrinum | |
| R. bakeri | |
| R. calendulaceum | |
| R. luteum | |
| Mollis group | |
| Exbury group | |
| 1Reported both as containing and not containing gossypetin. | |
In table 1, we have listed a number of yellow-flowered species of Rhododendron (and azaleas) according to their pigment class. Most of these data have been drawn from the published work of Harborne and his associates with some supplemental analyses, mainly on azaleas, from our own studies. We can see that the distribution of gossypetin does not appear to follow a definite pattern but occurs in both lepidote and elepidote species in several sections or series.
Common flavonols (e.g., quercetin glycosides) and flavones (e.g., luteoolin glycosides) have frequently been regarded as contributing yellow colors to flower petals. Actually, although these compounds are yellow in crystalline form, they do little but add body and substance to otherwise white or cream-colored petals. Only the rare flavonols with unusual patterns of hydroxylation (as gossypetin) or glycosylation (as in Rosa) are significant yellow pigments.
Other yellow pigments of the flavonoid group are the aurones (in snapdragon) and chalcones (in Dahlia) but these have not been found in Rhododendron or in the entire Ericaceae.
By comparing the structures of anthocyanins (I) and flavonols (III), we can see that there is a definite similarity. Indeed, there is a competition for the limited amount of precursors available for flavonoid synthesis. Less flavonol is produced in flowers with deep anthocyanin pigmentation, and the reverse is also true. However, in some plants, genes are known that increase the concentration of one class of pigments without affecting that of another. This situation in Rhododendron has not been studied.
There is little similarity between the structures of the flavonols and carotenoids. Theoretically, it should be possible to combine, by hybridization, the yellow flavonols and yellow carotenoids to enhance the yellowness of the progeny. However, Harborne (1965) could detect no carotenoids in the gossypetin-containing petals of R. campylocarpum and R. wardii. Thus, even though the chemical structures of these two classes of pigments are quite different, and there is no apparent competition for common precursors, their presence in rhododendron petals may be mutually exclusive. The non-colored flavonol quercetin is, however, found in most carotenoid containing petals.
What does all this mean to the rhododendron breeder? If the breeder's goal is confined to obtaining better yellow-flowered elepidote rhododendrons, he should continue to follow Leach's (1961) advice and concentrate on R. campylocarpum and R. wardii as parental stock to transmit yellow pigment. Although Leach's work was published before the pigment research reviewed here was published, his suggestions were well founded in experience. Whether the greatest yellow color intensity can be achieved by combining these cell-sap flavonols with plastid carotenoids derived from species like R. lacteum Franch, and R. dichroanthum Diels remains to be seen.
For the lepidote rhododendrons, the data in Table 1 should provide a starting point for breeding.
TOWARD A YELLOW-FLOWERED EVERGREEN AZALEA
The development of a yellow-flowered evergreen azalea has long been a goal of both professional and amateur breeders. In the following paragraphs, we will discuss the pros and cons of two approaches - both of which are theoretically possible as far as pigment inheritance is concerned.
1. Crossing Evergreen and Deciduous Azaleas.
The yellow pigments involved here are contained only in the deciduous species and are, as far as we know, all carotenoids. Very likely several different carotenoid compounds may be found in any given species or hybrid and it may be possible that certain species produce unique carotenoid pigments.
A major complicating factor is that the carotenoids are plastid pigments and chloroplasts (and other plastids?) are inherited maternally. The chloroplasts are cytoplasmic bodies and the unfertilized egg cell contains a large amount of cytoplasm, including the pro-plastids. The male gametes are generally considered to transmit little, if any, male cytoplasm during fertilization. We know there are nuclear genes that influence plastid development in some plants and some documented cases of male cytoplasm transfer, but the maternal cytoplasmic inheritance of plastids seems to be a characteristic in azaleas.
The junior author (Pryor, 1973) has provided ample evidence of maternal effects in crosses between evergreen and deciduous azaleas. When an evergreen plant was used as the female parent, the F1 progeny was entirely evergreen. When a deciduous plant was used as the female, the F, progeny was entirely deciduous although the leaves remained on the plant slightly longer than on the deciduous parent. Segregation for leaf character occurred only in the backcross (to deciduous) or F2 generation. However, our research was done exclusively on hybrids of Kurume x Mollis types and results from other combinations of evergreen and deciduous species might possibly be different.
The problem concerning the transmission of yellow plastid carotenoids in the first generation of such a cross is that the plastids are derived from the female evergreen parent and the genes (nuclear genes and/or plasmagenes) for enhanced carotenoid synthesis in the plastids are derived from the male deciduous parent. These male genes apparently have no control over the physiology of the evergreen plastids in the hybrid environment. Thus, there are little or no carotenoids in the hybrid petals and the flowers are essentially white.
In F2 or later generations and in progenies resulting from backcross generations, some evergreen plants are produced that contain a significant amount of chlorophyll in the flower petals. These flowers do not open normally and the green pigment remains throughout the life of the flower. Thus, even in evergreen plants that have segregated from repeated backcrosses to the deciduous species, there is a failure (or suppression) of activity of carotenoid genes from the deciduous parent. As mentioned above, these results are based only on Kurume x Mollis hybrids. Possibly results from the use of other evergreen females and different yellow-flowered deciduous males (such as R. luteum Sweet or R. austrinum Rehd.) would be different, especially if different carotenoid pigments were involved.
Based on present evidence, however, the only hope we see of achieving a yellow-flowered evergreen azalea by this route is the selfing or sib-crossing of some of the semi-evergreen yellow flowered segregates derived from repeated backcrossing to deciduous yellow-flowered types. The linkage between yellow flowers and deciduous habit in azaleas appears to be quite strong. Breaking that linkage will require the use of a wide range of parental germplasm, the possible restoration of fertility (by colchiploidy) of potentially promising hybrid parents, and a steadfast determination on the part of the breeder.
2. Crossing Evergreen Azaleas With Rhododendrons.
Numerous hybrids between Rhododendron subgenus Anthodendron (azaleas) and subgenus Rhododendron (rhododendrons) were listed by (Wilson and Rehder (1921) ). In all these hybrids, the azalea parent was a deciduous species of Sect. Pentanthera, but the azaleas were functional as both male and female parents.
Martin (1970) reported hybrids between rhododendrons and evergreen azaleas when the azalea was used as the male parent. He also reported 177 unsuccessful attempts to use evergreen azaleas as female parents in crosses with rhododendron.
Martin's results certainly do not rule out the possibility of successful hybridization involving evergreen azaleas as female parents. Most breeders work with a rather narrow range of species and/or cultivars and a wider trial of plant materials would be desirable. However, such crosses may be difficult to achieve.
In this connection we can discuss some of our own previously unpublished crossing data. We can assume, from past work, that the use of deciduous azaleas as females in crosses with rhododendrons is more likely to be successful than is the use of evergreen azaleas as females. When we used, as female parents, evergreen azaleas derived from F2 crosses of evergreen x deciduous azaleas or from backcross segregates of the F, to deciduous m a l e s not only were our crosses with rhododendrons successful but the hybrids also exhibited a significant degree of fertility. Thus, the infusion of genes from deciduous azaleas into phenotypically evergreen azalea hybrids appeared to significantly alter the crossability pattern.
Our recommendation for the creation of yellow-flowered evergreen azaleas by this scheme is to use white-flowered evergreen azaleas as female parents and yellow-flowered rhododendrons (such as R. campylocarpum and R. wardii or their hybrids) as male parents. The inheritance of flavonoids, like gossypetin, is usually such that hybrids contain a mixture of the flavonoids of both parents. The fact that evergreen azaleas possess genie systems that allow the production of gossypetin in leaves further strengthens the possibility of obtaining yellow-flowered, gossypetin-containing azalea-like hybrids in the first generation.
If desirable plants are not found in the first generation and the hybrids are sterile, the restoration of fertility by colchicine-induced chromosome doubling could be attempted. Selfing or backcrossing the fertile hybrid may then produce segregating progenies, among which a diversity of plant types might be found.
The production of gossypetin is controlled entirely by nuclear genes and the complications that may be encountered in dealing with plastid carotenoids are avoided in the above scheme. Although the use of rhododendron parents having carotenoid-yellow flowers might give the desired results, we believe the chances are slim indeed.
LITERATURE CITED
Harborne, J. B., 1962, Plant polyphenols. 5. Occurrence of azalein and related pigments in flowers of Plumbago and Rhododendron species. Arch Biochem. Biophys. 96: 171-178.
Harborne, J.B., 1965, Plant polyphenols XV. Flavonols as yellow flower pigments. Phytochemistry 4: 647657.
Harborne, J. B., 1969, Gossypetin and herbacetin as taxonomic markers in higher plants. Phytochemistry 8: 177-183.
Harborne, J. B. and Christine A. Williams, 1971, Leaf survey of flavonoids and simple phenols in the genus Rhododendron. Phytochemistry 10: 2727-2744.
Leach, David G., 1961, Rhododendrons of the world. Charles Scribner's Sons, N.Y. 544 pp.
Martin, Alexander C., 1970, Breeding azaleadendrons. Quart. Bull. Amer. Rhod. So. 24 (1): 39-41.
Pryor. Robert L., 1973, Hybridization between evergreen and deciduous azaleas. Quart. Bull. Amer. Rhod. Soc. 27 (4) 212-214.
Wilson, Ernest Henry and Alfred Rehder, 1921, A monograph of azaleas (Rhododendrons subgenus Anthodendron). Pub. Arnold Arb. No. 9, 219 pp.
by Elliot Garner
