QBARS - v29n3 The Flavonoid Chemistry of Our Native Azaleas: Uses in Classification
The Flavonoid Chemistry of Our Native Azaleas:
Uses in Classification
Bruce L. King
1
, Samuel B. Jones
1
and Fred C. Galle
2
1
University of Georgia,
2
Callaway Gardens
|
||||||||||||||||||||||||||||||||||||||||||||
In a recent article, King, Galle and Jones (1972) presented a preliminary report on a study then being initiated on the flavonoids from the leaves of the Azaleas native to the United States. The present article reports the completion of that study which demonstrated that flavonoids are useful in their classification. The project was undertaken with the cooperation of Callaway Gardens; it was supported by a grant from the Ida Cason Call-away Foundation, a Dissertation Improvement Grant from the National Science Foundation and by the Department of Botany, The University of Georgia. Since Callaway Gardens at Pine Mountain, Georgia has a large collection of most species of native Azaleas, a supply of leaf material was readily available. In addition to the species material, a number of hybrids are also in their collections. This provided a unique opportunity to compare the flavonoid patterns in the leaves of hybrid Azaleas with parental patterns. In addition, field collections from the southeastern United States and samples supplied by several other gardens were used. The technical details of this project are being prepared for publication elsewhere but we would like to present a general summary of our work.
Sleumer (1949) has grouped Azalea species into two sections of the subgenus Pentanthera (Table 1). The section Pentanthera is the largest with 19 species. Sixteen of these are found in the United States while the other three are Old World species. The section Rhodora consists of five species only two of which occur in the United States; Rhododendron vaseyi and R. canadense . The other three are restricted to Asia.
We found with only a few exceptions, that each species of native Azalea has its own characteristic set of flavonoid compounds or "fingerprints" varying in number from 21 compounds in Rhododendron austrinum to five in R. vaseyi (Fig. 36). A total of 58 different flavonoids were found in the group. These 58 compounds fall into five flavonoid classes: Flavonols, dihydroflavonols, dihydrochalcones, flavanones and chalcones. Most of the flavonoids had a variety of sugars attached to various positions of the molecule. Such flavonoid compounds are referred to as glycosides.
On the basis of similarity of flavonoids, the Azaleas of the section Pentanthera can be grouped into five natural alliances of closely related species. Chemically, members of the pink-white flowered Rhododendron canescens - R. nudiflorum - R. roseum alliance are characterized by the presence of eriodictyol, asebotin and 2', 6',4-trihydroxy-4'-methoxydi-hydrochalcone. The latter two compounds belong to a class of flavonoids called dihydrochalcones. In Rhododendron canescens these compounds occur in the majority of the populations examined. They are, however, replaced in certain southeastern Alabama, south Georgia and southwestern South Carolina R. canescens populations by two unidentified dihydrochalcones which have been designated as compounds 48 and 49.
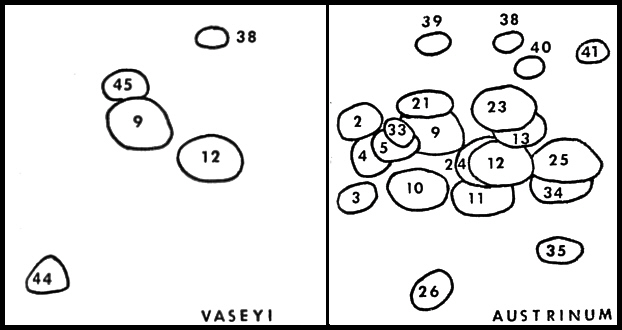
|
|
FIG. 36. Flavonoid profiles of
Rhododendron vaseyi
(left) and
R. austrinum
(right). The
profiles are schematic representations of 2-dimensional paper chromatograms. Paper chromatograms were developed in the long direction with TBA (tert-Butyl alcoholacetic acid- water, 3:1:1 v/v/v) and in the short direction with 15% acetic acid. R. vaseyi and R. austrinum have compounds 9, 12 and 38 in common. All species of native azaleas contained compound 9 (quercetin 3-0 rhamnoside). |
Species of the Rhododendron speciosum-R. prunifolium-R. calendulaceum-R. bakeri alliance are highly variable in flower color with yellows, oranges and various shades of red. While flavonoid profiles are quite distinct for each species a unifying chemical character seems to be the flavanones, farrerol and farrerol 5, 7-0-diglucoside found in all members of the alliance except the tetraploid R. calendulaceum . Plants of R. calendulaceum examined contained asebotin, a characteristic of the pink-white flowered R. canescens-nudiflorum-roseum alliance. This might suggest that R. calendulaceum was derived by hybridization of R. bakeri with an ancestral pink flowered species. Further investigations are needed on this interesting problem. Morphologically, R. calendulaceum belongs in the alliance with R. bakeri, R.prunifolium and R. speciosum and Willingham (1973) has presented evidence that R. calendulaceum is conspeeifie with R. bakeri . In addition to farrerol, individuals of R. prunifolium and R. bakeri were found to contain elaborate compounds 48 and 49 which were characteristic of the three southeastern populations of R. canescens . This suggests a close relationship between the R. speciosum-prunifolium-calendulaceum-bakeri and the R. canescens-nudiflorum-roseum alliances.
The Rhododendron alabamense-R. arborescens and the R. atlanticum-R, viscosum-R. corgi-R. serrulatum-R. oblongifolium alliances are closely related morphologically and chemically. Members of both alliances are white-flowered and lack the dihydrochalcones and flavanones characteristic of the previous two alliances. Rhododendron alabamense and R. arborescens are considered together as an alliance because of their similar flavonols and glycosylation patterns (glycosylation refers to substitution of sugars on the flavonoid molecule). In addition both species have an identical unidentified flavanone.
In addition to the absence of flavanones and dihydrochalcones, the
Rhododendron viseosum - cory i- oblongifolium - serrulatum
- atlanticum
alliance could be characterized by the presence of three myricetin 3-0-m onoglycosides. The presence of these monoglycosides here and their absence in
R. arborescens
and
R. alabamense
supports separation of these two groups as alliances, albeit, closely related.
Of particular interest is the Rhododendron austrinum - R. occidentale - R, luteum alliance. Rhododendron austrinum (yellow flowers) is restricted to the southeastern United States and R. occidentale (pink-white flowers) occurs on the west coast. The yellow-flowered R. luteum is the only known European Azalea. Surprisingly these three species, despite their widely different geographic areas, have in common a group of rare flavonoids called 5-0-methoxyflavonols. Such compounds have only been found in very primitive flowering plants (Harborne and Williams, 1971).
Rhododendron vaseyi and R. canadense of the section Rhodora differ morphologically, have different chromosome numbers and quite different flavonoid patterns. Interestingly, the section Rhodora differs from the section Pentanthera in flower morphology but there is no significant difference in the kinds of flavonoids found in the two sections. These two sections form the subgenus Pentanthera (Azaleas). The Azaleas lack a compound called gossypetin which, according to Harborne and Williams (1971) is found in all other subgenera of Rhododendron.
The native Azaleas of North America are considered taxonomically difficult. This taxonomic difficulty is, as Rehder (1921) and Skinner (1961) suggest, due to the great morphological variability of the species along with the existence of relatively few good "key characters." The flavonoids are of considerable value in solving some classification problems. It would not be practical to identify the native Azaleas by flavonoids alone but they are most useful in arranging the species into natural groups reflecting their biological relationships. Flavonoids are less variable in the Azaleas than other features such as flower color or leaf pubescence. If flavonoid information is correlated with the morphological features of Azalea taxa, it becomes much easier to place boundaries on species or to decide what is a good species and what is not. For example, Rhododendron oblongifolium , R. viscosum and R. serrulatum are extremely similar in most features but differ to some extent and show considerable variation. The flavonoids of these species are identical. This suggests that these three taxa might best be treated as a single species with three subspecies. By such a process of correlating morphological features with flavonoid chemistry a much better taxonomic treatment of the native Azaleas can be developed. It should also be mentioned that Solymosy (1974) has suggested that R. coryi is conspecific with R. viscosum .
As Galle (1967) has pointed out, another source of difficulty in the native Azaleas has been the blurring of morphological boundaries of species by natural hybridization. Where two or more species grow in the same general area and have overlapping flowering periods, natural hybrids are likely to be common. The species are genetically isolated from each other by differences in ecological habitat requirement, time of flowering and by geographical distribution. Interestingly, we have noted that hybrids often occur in intermediate habitats between those of the two parents. Natural hybridization is also associated with man-related perturbations of the habitats whereby man created unusual habitats where the hybrids could survive. Obviously, the occurrence of natural hybrids between two or more species can cause problems in identification. With hybrids between Rhododendron canescens and R. austrinum such problems are particularly acute since the only major morphological difference between the two species is flower color. Rhododendron canescens with, pink-white flowers, and R. austrinum , with yellow flowers, are quite different in flavonoids. The hybrids can usually be identified chemically since they contain some compounds of both species. With some individual plants it is impossible to determine whether or not that plant is a hybrid or a "pure" species.
The study of a hybrid colony between Rhododendron canescens and R. austrinum in southern Alabama indicated that most of the natural hybrid plants present were backcrosses. A backcross is produced when an F1 hybrid is crossed back to one of the parental species. It was also found that the biosynthetic pathways to many flavonoids were in same way disrupted suggesting that many genes of the two species do not function well mixed together in a hybrid. This information, along with the observation that natural hybridization occurs only in areas of intermediate or disturbed habitat, suggest gene flow between the two species is restricted. Therefore both species can retain their identity as species. This hypothesis is supported by the observation that populations of R. canescens and R. austrinum , collected from throughout their geographic ranges, were consistently distinct in their flavonoids. Furthermore, there is no evidence, other than the presence of occasional hybrid swarms, that these two species are merging together.
In summary the information obtained from this project has aided in solving several of the taxonomic problems in Azaleas, and increased our understanding of the group. Hopefully it will form the basis for a projected taxonomic revision of the subgenus Pentanthera. Such a taxonomic revision is badly needed as there is a great deal of confusion regarding the proper classification and treatment of our native Azaleas.
REFERENCES
Galle, F. C., 1967, Native and some introduced Azaleas for southern gardens: kinds and culture, Horticulture Magazine, 46:2-28.
Harborne, J. B. and C. A. Williams, 1971, Leaf survey of flavanoids and simple phenols in the genus Rhododendron, Phytochemistry, 10:2727-2744.
King, Bruce L., Fred C. Galle and Samuel B. Jones, 1972, A chemical technique applied to the study of our native azaleas, Quarterly Bulletin American Rhododendron Society, 26:275-276.
Rehder, A., 1921, The Azaleas of North America, In: E. H. Wilson and A. Rehder, A monograph of azalea, Publ. Arnold Arboretum, No. 9. pp. 107-196.
Shinners, Lloyd H., 1961, Rhododendron Coryi (Ericaceae), A new Reprint Series from Southeastern Texas, Castanea, 26:156.
Skinner, H. T., 1961, Classification of the native American azaleas, Proc. International Rhododendron Conference, the American Rhododendron Society, Portland, Oregon, May 11-14. pp. 81-86.
Sleumer, H., 1949, Ein system der gattung Rhododendron L. Bot. Jahrb. 74: 511-553
Solymosy, S. L., 1974, Notes on Rhododendron coryi Shinners, Quarterly Bulletin American Rhododendron Society, 28(3): 188-189.
Willingham, Frank F., 1973, Variation in Rhododendron calendulaceum (Michx), Ph.D. dissertation, Wake Forest University, Winston-Salem, N.C.
