QBARS - v30n4 Salt Damage and Its Interdependence with pH
Salt Damage and Its Interdependence with pH
Carl A. Deul, Northridge, California
Growing rhododendrons in areas where the pH of the growing medium and irrigation water is higher than 7.0 often presents more problems than chlorosis. In Southern California our irrigation water is often not only alkaline, but contains substantial amounts of dissolved mineral salts including boron. Salts are defined as chemical substances that are compounds of metallic and non-metallic elements. Sodium chloride or common table salt, is the one everyone is most familiar with. Typically these amounts are near the levels where care must be exercised in irrigation practices. Calculations of the sodium absorption ratios were made on our local water supplies and along with the analysis were compared with the standards for irrigation water quality outlined in the U.C. publication "Growing Azaleas Commercially" pp. 40-45. In all cases, with the exception of a few cases, the local water sources were adequate for Azaleas. Very often the gardener discovers burned leaf margins a short time after a mild acid fertilizer has been applied. Spectrographic analysis of the burned leaves shows excessive amounts of boron and manganese. Chemical analysis of the fertilizers showed the presence of these elements, but not at levels that should present a problem.
This situation has been a puzzle to us until very recently. The understanding lies in the relationship between the pH of the growing medium and the dissolved mineral salts in the irrigation water.
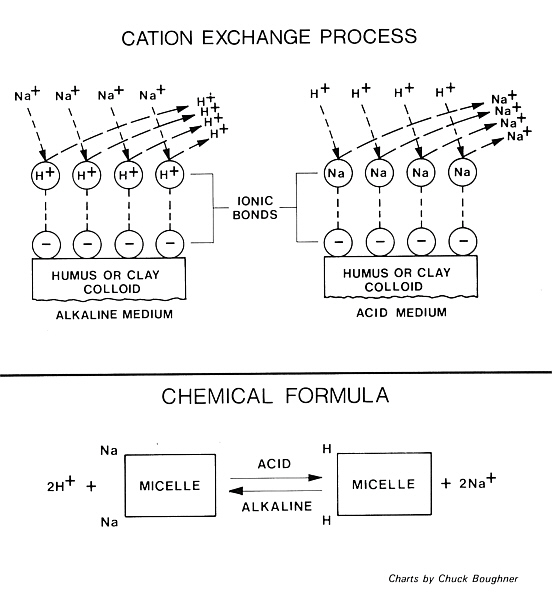
Whether the growing medium is a loam soil or a peat soil it is composed of many small particles called colloids. These particles are negatively charged on their surfaces due to their molecular structure. The negatively charged regions or radicals are called micelles.
When the growing medium and the irrigation water are acidic (less than 7.0 pH), hydrogen ions, which are positively charged, attach themselves to the micelles. Some of the metallic ions such as sodium, calcium, magnesium and manganese do so also, but to a lesser extent. If the growing medium and irrigation water are alkaline (greater than 7.0 pH), many of the hydrogen ions attached to micelles are replaced by the aforementioned metallic ions. In addition, the solubility of boron salts are less under alkaline conditions and they accumulate near the surface of the growing medium.
The reaction is reversible and when an acid fertilizer is applied the metallic ions and salts are released along with the boron and they become available to the plant roots in dangerously large amounts. To overcome this problem, we need to institute a program of frequent application of sulfur or acidified irrigation water so that the growing medium never rises above 7.0 pH for any length of time.
It is interesting to note at this point that the commonly recommended method of removing salts by leaching is almost worthless if the pH of the leaching water is 7.0 pH or greater. Leaching water should be acid in order to be effective.
|
REFERENCES:
"Growing Azaleas Commercially'' Editors: Anton M. Kofranek and Roy A. Larson, Division of Agricultural Sciences, University of California publication No. 4058, pp. 40-45.
"Western Fertilizer Handbook'' produced by: Soil Committee California Fertilizer Association, pp. 26-28.
"The Nature and Properties of Soils, Nyle C. Bradey, pp. 30-35, 71-101
