QBARS - v34n4 A Rhododendron Leafhopper- Field Recognition and Habits
A Rhododendron Leafhopper - Field Recognition and Habits
A. G. Wheeler, Jr. and K. Valley
Bureau of Plant Industry, Pennsylvania Department of Agriculture
Harrisburg, PA
Abstract
The leafhopper
Graphocephala coccinea
inserts pouch - like eggs in leaves of rhododendron. Its feeding does not normally injure foliage of ornamental azaleas and rhododendrons, but its eggs may disfigure the leaves and attract the attention of growers. Tiny holes sometimes appear in leaves when tissue drops from areas of egg deposition. A brief description of the adult and immature forms of the leafhopper is provided, and its seasonal history on ericaceous shrubs is summarized.

|
|
Fig. 2. Close-up of
G. coccinea
egg
Photo by James F. Stimmel |
|
||
|
Leafhoppers are among the most common insects found on ornamental plants. These insects, which are related to cicadas, spittlebugs, and treehoppers, extract sap from plants by means of a beak or proboscis. Of the estimated 3,000 species known to occur in North America, only a few ever attract a grower's attention. Most leafhoppers associated with ornamentals feed without producing noticeable damage or transmitting plant disease - and many are minute (3 mm long or less) and of modest coloration. A notable exception is a handsome species found on azaleas and rhododendrons. Graphocephala coccinea (Forster), brilliantly colored and 8 to 9 mm long, is one of our most conspicuous leafhoppers.

|
|
Graphocephala coccinea
Photo by James F. Stimmel |
G. coccinea , sometimes called the redbanded leafhopper, occurs throughout most of the U.S. east of the Rocky Mountains, as well as in Ontario and Quebec, Mexico, and Central America. A similar-appearing species associated with rhododendrons is G. fennahi Young, described in 1977 from several eastern states. This latter leafhopper has been much confused with coccinea but was recognized as a distinct species mainly on differences in the male genitalia. It is G. fennahi that has been introduced (probably with rhododendron nursery stock) into the Pacific Northwest and the Kew Gardens of London. The records of G. coccinea from rhododendron in France, the Netherlands, and Switzerland probably also should refer to G. fennahi . Because introduced insects may become pests even though they are innocuous in areas where they are native, European horticulturists are carefully observing the habits of G. fennahi on ornamental rhododendrons.
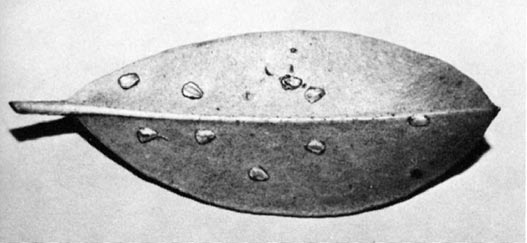
Despite its striking appearance G. coccinea is not a well-studied leafhopper. It is known from more than 50 species of plants and to injure ornamental shrubs like forsythia; but only incomplete, sometimes conflicting, information is available on its seasonal history. The life cycle can be completed on both azaleas and rhododendrons, but on ericaceous shrubs eggs are laid in the more thickened leaves of true rhododendrons or mountain laurel. The pouch-like eggs, appearing as swellings or bumps on foliage (Fig. 1, 2), probably have puzzled many growers. After the eggs have hatched, scars remain at the sites of oviposition. In succeeding years a grower may find mysterious holes in rhododendron leaves where tissue has dropped from areas of egg deposition. In this paper we provide information allowing a field recognition of both immature and adult forms of the leafhopper and an understanding of its life history and habits.
Field Recognition of Graphocephala coccinea
Adults of G. coccinea are among the most strikingly colored of the North American leafhoppers. Brilliant, alternating bands of red and green on the outer pair of wings contrast vividly with the yellow legs, face, and top of the head. A distinct black band is present at the junction of the face and the top of the head.
In sharp contrast to the adults, body color of the nymphs is a pale yellow. The first two nymphal stages also display the distinct black facial band found on the adult, and in addition, conspicuous black bands are present on the dorsal (top) surface of the thorax and abdomen (the two body divisions behind the head). The last three nymphal stages lack black bands. The nymphal stages also lack functional wings, and thus, cannot fly.
Seasonal History
We studied the seasonal history on Rhododendron maximum, an evergreen azalea (undetermined cultivar), and Kalmia latifolia (mountain laurel) growing in a cemetery at Harrisburg, Pa. Our observations, made at irregular intervals from 1976 to 1978, indicated that this leafhopper produced only one generation annually. This agreed with the findings of a British researcher (who actually had studied G. fennahi) but conflicted with those of Herbert Osborn, at the time North America's foremost authority on the leafhoppers. In 1912 he had reported two generations for G. coccinea. We therefore made more detailed observations in 1979, taking samples from leafhopper populations on the three ericaceous plants at weekly, or at least biweekly, intervals from April to October. All specimens reared from ericaceous plants refer to G. coccinea rather than G. fennahi, which the Europeans have called the "Rhododendron leafhopper."
The time of the first egg hatch in spring was determined by finding, and then marking, rhododendron leaves on which over wintering eggs had been laid. These leaves were then observed every few days beginning in mid-April. Although eggs of G. coccinea had begun to hatch as early as April 19 in 1976 and April 22 in 1977, they did not begin to hatch until May 7 in the comparatively late spring of 1979.
Upon hatching the tiny immature leafhoppers, or nymphs, remove sap from foliage of their host plants, usually feeding on the lower leaf surfaces. There are five nymphal or immature stages. By mid-May the populations consisted mainly of 3rd-and 4th-stage nymphs. At this time, the leafhopper was most common on the evergreen azaleas and mountain laurel even though over wintering eggs had been most numerous on rhododendron. Apparently the early-stage nymphs leave rhododendron to feed on the more succulent foliage of azaleas and numerous other shrubs and herbaceous plants.
During the last week of May and the first few days of June, 4th-and 5th stage nymphs were present. The first adults appeared during the second week of June, but late-stage nymphs were still present into July. We first observed mating in early July and found newly deposited eggs in rhododendron leaves by mid-July. These eggs were found in leaves of the current season, thus marking the beginning of a second generation. The first nymphs of this second brood were found July 16 on a wild Rubus sp. near the sample area and were found a few days later on azalea and mountain laurel. Third-and 4th-stage nymphs were present in early August, with 5th or last-stage nymphs making up most of the population by August 15. Adults of the second brood began to mature during late August, although late-stage nymphs could still be found by mid-September. Eggs (those that will over winter) apparently were not laid until mid-September or later. Adults are common on ericaceous shrubs, as well as other plants, during October or until the first killing frosts. They were present until late November in 1979.
Natural Enemies
In our collections we found only one natural enemy of G. coccinea, an egg parasite. Leafhopper eggs collected in late March, early June, and mid-July were parasitized by a small black wasp of the family Mymaridae (Gonatocerus novifasciatus Girault). The wasp adults chew a small irregular hole in the egg when they emerge. This irregular hole contrasts sharply with the longitudinal slit produced by the leafhopper nymph when it emerges from the egg. Parasitized eggs have a darkened appearance; un-parasitized eggs are yellowish. Only one wasp emerges from each parasitized egg.
Economic Importance
The feeding of this leafhopper does not seem to produce the stippling or chlorotic appearance of foliage that is characteristic of leafhopper injury to apple and several other plants. Despite the large populations of G. fennahi that breed on rhododendrons in England (40 or more cast skins have been found on a single leaf), no economic damage has been observed. Only a few terminal leaves were reported to show yellow spots on the upper surface. We observed no visible injury to azalea or rhododendron foliage in our studies.
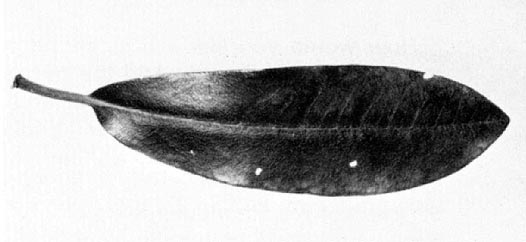
It is the eggs, and the scars left after they hatch, that may attract a grower's (or nursery inspector's) attention. Eggs deposited just under the upper or lower epidermis of rhododendron and mountain laurel leaves appear as prominent, oval swellings. They are especially noticeable when several are inserted in a single leaf. After egg hatch, a scar remains on the upper surface where the egg had been inserted. This scar progressively darkens, and in succeeding years leaf tissue may drop from the scars, leaving tiny roundish holes in the foliage (Fig. 3). Studies of G. fennahi in England indicate that this species deposits eggs in sepals of the flower buds rather than in leaves
Generally not enough eggs are laid in rhododendron leaves to detract from the appearance of the plants, so control measures are not usually necessary. We feel it is important, though, for a grower to recognize this unaesthetic appearance of rhododendron foliage for what it is - holes resulting from leafhopper oviposition in previous seasons. The tendency would be to blame some chewing insect for this damage. Caterpillars, weevils, and other chewing insects do feed on rhododendrons and may warrant control. Usually, however, they have irregular holes at the edges of the leaves or larger holes in the interior of leaves.
Acknowledgements
We thank James F. Stimmel of our laboratory for the excellent photographs. He and our colleague Thomas J. Henry kindly reviewed the manuscript. Robert L. Furniss, Portland, Oregon, called our attention to literature on G. fennahi.
by Elliot Garner