JARS v36n2 - Rhododendron Borer: A Worthy Competitor
Rhododendron Borer: A Worthy Competitor
John W. Neal, Jr.
Florist and Nursery Crops Laboratory
USDA, SE, ARS Beltsville, Maryland
Rhododendrons, when grown as fine specimen plants at home, in an arboretum, in wooded lots or in sunny nurseries, are subject only to a limited number of debilitating insect and disease problems. This makes the risk of growing this spectacular flowering ornamental shrub relatively low. The rhododendron, because of its inflorescence display, is given a high degree of care by its devoted owner; it also happens to be a preferred host of the larvae of a clearwing moth borer. Both grower and insect seek fundamental but different needs in the rhododendron - thus the competition.
One of the more destructive insects of the rhododendron is the rhododendron borer, Synanthedon rhododendri (Beutenmuller), a clear-wing moth of the family Sesiidae. This family includes other aggressive plant pests such as the dogwood borer, lilac borer, peach and lesser peach tree borers, viburnum borer, ash borer and squash vine borer. These colorful day-flying moths strongly resemble many species of wasps and are frequently mistaken for them by the nurseryman or rhododendron specialist.
The rhododendron borer, one of the smallest clearwing moths, was first described in 1909 from specimens collected at Cheltenham, Pa. It was not until after the initial taxonomic description that the economic importance of the species was recognized. A heavy infestation, in which many shrubs were killed, was later reported in the Botanic Gardens of Brooklyn, NY, in 1918.
The biology of this moth has been difficult to study because of its wasp mimicry, and a lack of adequate infestations. I fortunately had access to two commercial nurseries in Maryland, which provided splendid cooperation and ample material to study this colorful moth. The recent availability of commercial synthetic clearwing moth sex lures and sticky traps also helped in determining the correct flight time for males of this species. The observation and biological data reported in this study pertain to Maryland, and an appropriate time shift should be made for more northern and southern latitudes.
Distribution: In 1946, the range of the rhododendron borer was reported as the Atlantic Coast States, from Pennsylvania to Kingston, Rhode Island (Englehardt, 1946). Records were obtained primarily by rearing the adults from infested rhododendron cuttings.
The use of pheromone traps has rapidly expanded the known distribution of rhododendron borer north to Middlesex, Massachusetts, south to Clemson, South Carolina, and west to Ohio. This insect has not been collected from traps at Urbana, Illinois, or from Puyallup or Olalla, Washington. A pheromone trap kit is sold by CONREL (110 A Street, Needham Heights, MA 02194); the pheromone also attracts males of the lilac borer, oak borer, viburnum borer and peach tree borer throughout the growing season. These traps are educational to use because they allow observation of insects which have previously evaded us. These traps, however, do not provide control of this pest.
Hosts: Rhododendrons are preferred hosts for oviposition; however, mountain-laurel ( Kalmia latifolia L.) and deciduous azaleas, when grown with heavily infested rhododendrons, also become infested.
Life History and Field Observations
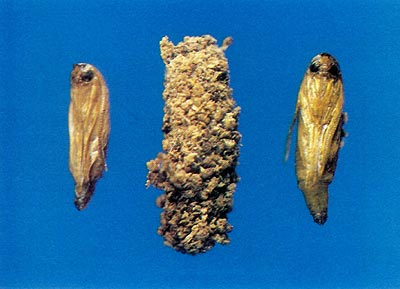
Prior to emergence as adults, the pupa orients itself towards a circular paper shell window (Fig. 1) which it had prepared as a larvae. A cut is made through the pupal case with a distinct cutting ridge on the anterior margin of the head (Fig. 2). The circular window is ruptured, and the pupa partially protrudes from the opening. The adult emerges from the pupal skin by rupturing a thin suture along a median line on the dorsum (top) of the head. Emergence occurs within a few minutes during the morning while the humidity remains high. After the wings are expanded and have hardened, the adults are capable of flying but generally spend time resting. Pupal skins will generally remain on the plant for several weeks; their presence can give an idea of the rate of infestation and concealed damage.

|
|---|
Fig. 1 Pupal case and parchment window prepared for adult emergence. |
|
|---|
Fig. 2 Pupa with cutting ridge and pupa case covered with larva excrement (frass). |
Both sexes are sexually mature at emergence and the female mates on the first day. When the female is receptive for insemination, she begins to secrete a volatile fluid from a gland near the ovipositor at the end of the abdomen. Secretion of the fluid with the extension of the ovipositor is referred to as 'calling' and the volatile material is called a pheromone. The calling female generally is observed near the margin of an upper surface of a leaf on the outer branches of the plant. Most frequently she will alight on the shaded portion of the leaf. A responding male announces his arrival by approaching from the rear and striking the calling female on the dorsum of her abdomen. The female flies to a nearby leaf and is followed by the male. This male behavior is called a precopulatory strike and results in the female to cease calling. I have observed as few as one and as many as 6 precopulatory strikes on the same female. Adults do not walk on leaf surfaces or alight on the under sides of leaves, however the female does walk on the trunk and branches after mating when searching for egg laying sites. Females are mostly active on bright, sunny warm days. I have observed that flights and calling activity decrease whenever the sun is obscured by clouds. Flight activity generally occurs from mid-morning to mid-afternoon.
After mating, the female moves into the inner canopy of the plant and seeks sites for egg laying. Sites frequently chosen are old pruning scars, narrow V-crotches, scars where the inflorescence was broken off while still green, and the most desirable site - old larval feeding galleries. The highest concentration of borer eggs I observed was an area of protruding pupal skins. The eggs are tucked deeply into cracks and bark crevices and are large enough to be barely visible to the trained observer. Adults do not feed and live no more than a day or two.
A breakthrough occurred in 1974 when USDA chemists and entomologists, working together, isolated and identified the 2 major isomeric chemical components of the pheromone system of the female peach tree borer and lesser peach tree borer. It is uniquely coincidental that the commercially available synthetic pheromone attracts males of other species of clearwing moths as well as the peach tree borer. Scientists are now using synthetic sex lure to obtain more accurate records of species distribution and times of flight. With this knowledge, insecticide applications may be applied at critical times, thus greatly increasing the efficacy of any given spray application.
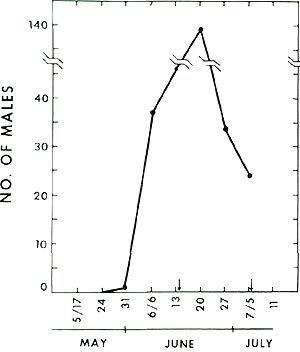
The synthetic sex lure available from CONREL was used successfully in Maryland during 1978 through 1980 to determine the flight period of this borer. Prior flight data are quite old and questionable because infested stems were cut and brought into the laboratory to allow for pupation and adult emergence. The rather uniform heating of rooms accelerated development and led to the early spray recommendations (late May and early June). Trapping data I have obtained clearly show that the male flight in Maryland begins in the last week of May and continues into the first week of July - Memorial day to July 4th (Fig. 5).
|
|---|
Fig. 5 Male flight profile at Beltsville, Maryland. |
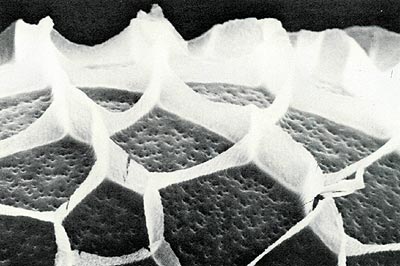
The borer egg, when examined with a scanning electron microscope (Fig. 6), has hexagonal and polygonal reticulations on the surface, which are remnants of the site of origin in the ovary. The egg surface has many small indirect openings called aeropyles which permit the exchange of gases. The sides of the egg become slightly depressed with maturity. Eggs incubated at 25°C and 70% RH hatch in 11 to 13 days which may suggest a slightly longer period on the shrub with fluctuating spring temperatures.
|
|---|
Fig. 6 S.E.M. photograph of the surface of the rhododendron borer egg that shows the polygonal reticulations and the aeropyles. |
Upon hatching, the larva immediately seeks seclusion from desiccation and predators and begins to chew an entrance under the bark, preferring roughened bark areas near old wounds or borings with new callous tissue. Larvae immediately begin feeding and continue until the onset of severe cold weather. During growth and development the larva undergoes a series of molts, enlarging each time. In early December the larva is not quite mature and ceases to feed; it spins a light, silken chamber in which it rests until early to mid-March when it resumes feeding. I have observed large amounts of fresh frass (fecal material and wood particles) at the base of infested plants as early as March 21 of 1978, 1979, and 1980 at Beltsville, Maryland. The larvae continue to feed until late April or early May, at which time they cease feeding and evacuate their gut of all food contents. The larvae then chew their way to a site under the bark suitable for emergence. The sides of the gallery at the pupation site are enlarged and the walls are made very smooth. The larva chews on the inner bark, creating a round opening and leaving a thin outer layer of bark intact (Fig. 1). This window is not obvious from the outside, but birds are able to detect the presence of pupae. The larva spins a thick, silken cocoon in which small pieces of wood particles are incorporated (Fig. 2).
The pupa, averaging 6 to 10 mm in length, is yellow-tan, in color, darkening to black prior to adult emergence. The posterior end of each abdominal segment on the top side of the pupa has a well-developed double row of spines which it uses to push itself forward at time of emergence.
The overall appearance of adults from above is a small clearwing moth 10 to 15 mm; the body is dark metallic blue with abdominal segments 2, 4 and 5 having a pronounced yellow band (The dogwood borer is the same size but has yellow bands only on segments 2 and 5). Closer examination will reveal shiny coppery color scales on the veins of the wings, brilliant white on the underside of the thorax, and anal tufts which are broadly fan shaped with white margins on the male (Fig. 3) and well tufted with some whitish scales on the female (Fig. 4).

|

|
|
|---|---|---|
Fig. 3 Male with fan tail. |
Fig. 4 Female with tufted tail. |
Varietal Preferences: In 1978 a large block of rhododendrons 5 to 7 years old, was examined and found to be heavily infested. Varieties were field lined in paired rows and the trunk and branches critically examined from the ground to the canopy for the presence of fresh frass and old larval galleries. Of 8 varieties examined, 72 percent of 'Purpureum Elegans' showed some sign of borer damage. 'Catawbiense Boursault', 'English Roseum' and 'Catawbiense Album' showed 33 to 40 percent damage (Table 1). R. catawbiense var. album and 'Blue Peter' had the lowest levels of damage; no variety escaped infestation. During the plant examination bird pecking damage was observed frequently at sites where there had been considerable larval activity.
| Table 1. Rhododendron Cultivars showing Rhododendron Borer Damage. | |||
|---|---|---|---|
Number |
Percent |
||
Cultivar |
Examined |
Infested |
Infested |
'Purpureum Elegans' |
61 |
44 |
72 |
R. catawbiense var. album |
327 |
25 |
07 |
'Catawbiense Boursault' |
80 |
27 |
33 |
'Nova Zembla' |
354 |
51 |
14 |
'Catawbiense Album’ |
42 |
17 |
40 |
'English Roseum' |
9 |
3 |
33 |
'Blue Peter’ |
133 |
12 |
09 |
'Gomer Waterer' |
57 |
10 |
17 |
1,063 |
189 |
18 |
|
Age of borer damage was not considered. |
|||
In 1979, nearly all plants in a large field planting 'Catawbiense Boursault' were infested. All plants were rogued out in mid-summer because of the severe infestation.
In 1978, 11 cultivars of mature deciduous azaleas planted for landscaping purposes were examined for borer damage. Eight cultivars were usually represented by 2 plants. Red letter, the only cultivar with 3 plants showed damage on each plant (Table 2). Other plants with damage were Madeleine', 'Balzac', and Primrose. In 1979, a long single row of 'Directeur Moerlands' was examined and the larger mature plants showed signs of borer damage. This information is reported on varieties which are susceptible under unusually high borer pressure.
|
Table 2. Mature Deciduous Azaleas Infested with
Rhododendron Borer |
||||
|---|---|---|---|---|
Plant Size 1 |
Infested |
|||
Cultivar |
No. 1 |
No. 2 |
No. 1 |
No. 2 |
White Swan |
2.05 |
2.87 |
- |
- |
'Firefly' |
3.25 |
2.67 |
- |
- |
'Madeleine' |
3.14 |
2.40 |
+ |
+ |
Orange ball |
2.75 |
3.30 |
- |
- |
Peach Sunset |
3.50 |
2.85 |
- |
- |
'Balzac' |
3.83 |
+ |
||
'Brazil' |
3.72 |
- |
||
'Berry Rose' |
2.45 |
2.72 |
- |
- |
Red letter |
3.96 |
2.93 |
+ |
+ |
Red letter |
3.95 |
+ |
||
Primrose |
2.25 |
2.69 |
||
'Klondyke' |
2.69 |
- |
||
1 circumference at 12 inches |
||||
Control: Lindane, and Dursban 1 are registered for use on rhododendrons for control of the rhododendron borer larva. Current recommendations for making 2 to 3 spray applications in May and early June are based on emergence of adults under laboratory conditions. The capture of naturally occurring adult males in sex lure traps shows that sprays should be applied in mid-June and early July to be effective against newly hatched larvae. An experiment was designed to determine effectiveness of timing of applications of insecticides for early larva control at Beltsville, MD. Lindane, Dursban and Orthene were applied either at 1) mid-flight and 2) mid-flight and end of flight (Table. 3) based on trap data. The results show that recently registered Dursban provides better control than Lindane. Dursban was also recently registered for control of the dogwood borer.
| Table 3. Evaluation of Insecticides Based on Male Flight Data | ||||
|---|---|---|---|---|
Insecticides 1 |
No. of Appl. 2 |
No. of Plants Infested |
No. of Larvae |
Avg. No. Plant |
Dursban |
1 |
2/7 |
4 |
0.57 |
2 |
0/9 |
0 |
0.00 |
|
Lindane |
1 |
5/8 |
18 |
2.25 |
2 |
4/8 |
26 |
3.25 |
|
Orthene |
1 |
7/9 |
189 |
21.00 |
2 |
7/7 |
80 |
11.42 |
|
Untreated |
0 |
8/8 |
184 |
23.00 |
1 Label rates applied for use on woody ornamentals. |
||||
2 Plants treated June 15 or June 15 and July 6, 1979. |
||||
The borer has demonstrated varietal preference as seen earlier with 'Purpureum Elegans'. The slow selection process of hybrids for cold hardiness, color, and growth habits would make the selections for pest resistance very difficult.
The current practice by many growers of using a thin wire to destroy the larvae in galleries under the bark only exacerbates the condition by actually making additional wounds on the plant which are choice egg-laying sites.
The use of properly timed insecticide provides the most complete and thorough control. Do not waste spray by applying it to the leaves; thoroughly wet the stems, branches and trunk of infested plants. Add several drops of a liquid detergent to the tank for each gallon of spray mix to allow spray to penetrate the bark and crevices.
1 Dursban (=chlorpyrifos) has recently been approved by EPA for use to control the rhododendron borer. Dursban is a proprietary product of Dow Chemical Company. CAUTION: due to the ever increasing development of new rhododendron cultivars, applicators should test a small number of each cultivar for phytotoxicity before general use. This is a good practice with any new product. The use of trade names in this publication does not constitute a guarantee, warranty, or endorsement of the products by the U.S. Department of Agriculture.

