JARS v36n3 - Measuring Fertilizers and Salts in Rhododendron Composts
Measuring Fertilizers and Salts in Rhododendron Composts
A Method Used by John G. Lofthouse, Vancouver, B.C., Canada
Too Much "By Guess & By Gosh"
The feeding of rhododendrons from tiny seedlings to mature plants, and the suitability of media for rooting cuttings, is often subjected to chance. Sometimes results are good, often poor. In this age of instrumentation, we hobbyists have few scientific guides, other than our own experience. In this modern age we should have more than that!
Too Much Good Can Be Bad
Many millions of seedlings and cutting propagated rhododendrons have been killed by toxic chemicals in the growing or rooting compost. In some cases the grower was puzzled over the reason for their demise. "Toxic" in this article can also apply to the chemicals in fertilizers applied at too great a concentration.
Plants contain in their tissues many chemicals that are mainly absorbed by their hair roots, from substances in the soil. Healthy rhododendron plants and seedlings will have a heavier concentration of these chemical salts in plant and root cells than in compost surrounding their root systems. This higher solution of salts in the root hairs creates a "pull" on the water (osmosis) from the compost adjacent to the roots. Thus water and nutrients flow into the plant. Other factors being satisfactory, seedlings and plants flourish and cuttings normally root well.
Sometimes chemical concentrations in the soil increases to an amount greater than the plant roots contain. Water from the plant's root hairs flow out of the plant, causing desiccation of the plant cells, wilting, and if not corrected, eventual death.
Problems With Peat
While over fertilizing may be the main factor for this chemical damage, it is not necessarily always the cause. A number of years ago, the writer reported to an ARS convention in Seattle about toxic elements (chemical salts) appearing in peat. This is much more prevalent than generally realized. In a rooting compost, this spells trouble - delayed rooting and often death of the cuttings. Seeds planted in it suffer a similar fate. Instead of white vigorous hair roots, none form, or if they do, turn brown and die.
In rhododendron culture, seedlings and rooting cuttings are much more susceptible to mild toxic elements than more mature plants growing outside. The writer has a source of excellent sphagnum peat from a non commercial bog on Vancouver Island. It is a light brown, excellent texture, but has about four times the toxins (250 parts per million) that I consider allowable for a rooting compost. Luckily, most of this can be washed out. It then becomes one of the finest peats I have found.
Problems With Propatation
Contaminated peat causes many more problems in closed case propagators than under mist systems. Mist gradually helps to flush out these elements; in "closed case" they stay much the same. Propagators using either system would do well to follow the tests later described for testing peat.
Atoms & Ionization
Most fertilizers, a blend of nitrogen, phosphates, potash and other ingredients, are found in the soil in the form of chemical salts. When dissolved in soil water, the atoms and molecules become ionized; that is, take on an electrical charge. Some lose electrons and become positively charged, some gain electrons and become negatively charged. If two metals such as zinc or steel are placed in water or a saturated compost containing dissolved salts, ionization causes an electric current to flow in a meter connected between these electrodes. Generally, the higher the salt content, the higher the meter reads. Therefore, a meter that incorporates in its design two electrodes, as described, could be calibrated to read salts and/or fertilizer concentrations in composts or solutions.
Finding An Answer
The writer has found, that the common moisture meter, available at many supply stores, gives an excellent reading of chemicals in the soil. Though called a moisture meter, it is not. The meter's response is directly proportional to the increasing amounts of chemicals dissolved in the water.
These units consist of a sensitive electric meter connected to a slender probe that is inserted into the liquid or compost being tested. This probe, consisting of two dissimilar metals (here zinc and steel) indicates an electric current in the presence of acids and alkalines and their combinations (chemical salts) in a water solution. This is somewhat like an electric battery. Up to a certain limit, the higher the chemical content, the higher the reading.
An interesting experiment can be conducted. Placing the probe in distilled water gives no deflection on the meter. By adding a liquid fertilizer, drop by drop while stirring, you will note the indicator deflects upwards towards, and possibly past, the maximum. Salt, acidic juices, vinegar and bleaches, as well as chemicals in the soil will also make it deflect. Some meters are so sensitive that one grain of salt can be detected in an ounce of water. When measuring for chemical content, ignore the Wet-Dry markings on the meter.
From the foregoing, you can see that the proper operation of the meter depends on an adequate supply of clean water. Our pure mountain water in Vancouver provides no problem. Should your water be heavily mineralized or chlorinated, adjustments will have to be made. Heavily chlorinated water can be drawn several days ahead. The chlorine should evaporate off. If your water is not suitable, distilled or un-contaminated rain water could be used.
|
|
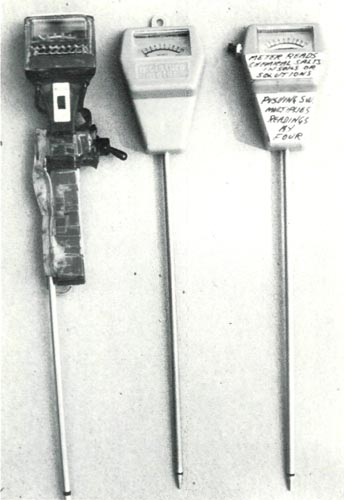
Fig. 1. Two different "moisture" meters. Unit
on left has a light meter incorporated. Center meter is preferred. (200 Micro Amp movement). On the right same meter after modification. This is the same meter as shown in Fig. 3. Photos by author. |
Selecting The Instrument
There are many "moisture" meters on the market. Refer to photograph #1. The instrument on the left is sold as a moisture and light meter. I have added a switch and use it as a salt indicator. The meter is too sensitive (@100 micro amps) to give practical readings. I prefer the meter in the center as purchased, then shown on the right as adapted. Current at 200 micro amps deflects it to full scale. It is made in Taiwan but has no manufacturer's identification. It claims to measure only moisture.
Another range of sensitivity is desirable. This will measure higher concentrations of fertilizer salts, such as tomato composts and other plants that are heavy feeders. These concentrations would drive the un-adapted meter off scale.
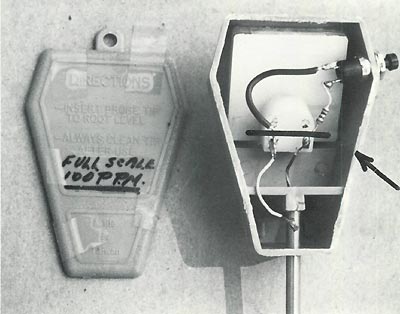
Figure 2 shows the installation of switch and resistor (Radio Shack, 100 ohms, ¼ watt). The miniature switch is spring loaded and contacts when pressed. (Radio Shack CAT. #275-1 547). Remove back of instrument for installation of above parts. As this is factory sealed along the edge, a sharp knife can be used to pry it open. It will not break if done carefully and can be glued back later. One half teaspoon of table salt dissolved in 5 Imperial gallons of water (6 U.S. gallons) will deflect the meter full scale. This is a full scale sensitivity of approximately 100 parts per million. Pushing the button multiplies the readings by four. The meter should be available at department, hardware, variety and plant stores. Try to obtain the same as illustrated, or it may not be satisfactory. Simpson-Sears Ltd., 4750 Kingsway, Burnaby, B.C., Canada, usually stocks it priced at $10.00 Canadian Funds, 1982 prices.
|
|
Figure 2. Meter with back removed. Parts above
black line (see arrow) have been added to give another range of readings when button is pushed. Resistor is 100 ohm, ¼ W. Regular range indicates salts at 100 parts per million. Second range 400 ppm. |
|
|
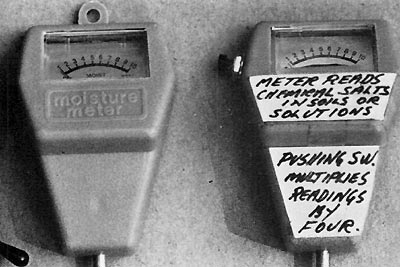
Figure 3. Meter on left before modification. On right
after. Labels applied by J. Lofthouse. Meter when received points to left. Author removed scale and adjusted needle 3/16” to left and made a zero reading. Camera angle does not show it pointing exactly on zero. This modification is not essential, but improves accuracy at bottom end. |
Proper Use
When measuring salt concentrations in composts, be sure medium is saturated with water as pure as possible. Salts in irrigation water will affect readings. Readings drop off after a few seconds because of film build up on steel shaft. First readings are the ones to take. Clean the shaft after each use, or readings will not be accurate. Do not leave in solution longer than necessary or tip will erode due to electrolysis. Dry promptly.
Testing Peats
With the peat sufficiently moist from comparatively pure water, compress some in your hand and insert probe. I would suspect peat (used for growing seeds and for propagation) to be contaminated, if reading is above 6 or 7 on the regular scale (60-70 parts per million). Sawdust, bark mulch and sand can be tested similarly.
Most soluble toxic chemicals can be flushed out with water. Place peat, etc., in a container with a screened bottom and apply sufficient water until peat tests within range. If you wish to prepare some for the future, place peat in a clean raised area and let the rain and snow do the job naturally.
Growing Rhododendrons In Containers
Where climatic conditions allow, many rhododendron enthusiasts are growing greater numbers of plants in containers. Because they take less room, more plants can be grown. Because of limited root space and better control of feeding, they generally bloom sooner. There are other advantages, such as better looking plants with improved foliage. For a number of reasons, they are best grown in an artificial medium. One that works well for me, and I urge you to try, is equal parts of peat and bark mulch. For small containers, screen out pieces of bark larger than ½". The bark we get here is either hemlock, spruce or fir. I am sure many other bark mulches are satisfactory. Although I don't like it as well, sawdust will do.
Variations in the mix will work, but reducing the peat means more watering. A good mix can be 1 part peat to 3 or 4 parts bark, if peat is scarce or expensive. To this is added a fertilizer mix. I make up and use the following:
16 oz. Osmocote 18-6-12, nine months formula.
6 oz. regular superphosphate 0-18-0.
6 oz. dolomite lime.
6 oz. gypsum.
1 oz. fritted trace elements.
Mix the fertilizer thoroughly.
Spring Potting
For semi mature or mature plants, potted up and to remain outside during spring and summer, use 1 lb. of this mix to 20 Imperial (24 U.S.) gallons of compost. For smaller quantities 1 rounded tablespoon to a U.S. gallon. Mix in thoroughly so no fertilizer "hot spots" develop. Mixing the pound of fertilizer with 1 gallon of perlite gives you visual indication of your mixing efforts. This formula will release fertilizer salts at approximately 70-100 parts per million after several weeks. Most rhododendrons, after juvenile stage, can be kept at this level for maximum growth during the growing season. Give additional top dressings as required. Let readings drop to approximately 10 parts per million during winter. Fall rains will help to achieve this level naturally if feeding is discontinued during the Fall.
Fertilizer sensitive plants should be given less fertilizer. Heavy feeders might benefit by more.
If potting is done in the fall and plants are to be carried over winter in a plastic house or greenhouse, use no more than 25% of the fertilizer. Even then, watch for toxic build up if plants are not watered, as often happens under glass or plastic during winter. Use your meter to measure toxicity and if readings are high, flush occasionally with water.
Measuring Fertilizer Levels In Containers
Because your plants are now growing in soilless cultures, the readings now on your meter are generally from added fertilizers. A falling off of the meter readings as time goes by, indicates a leaching of the fertilizers. Keep a record of your container readings, at the start, then every month through spring, summer and fall. Next year the extra fertilizer required can be judged by your proper meter monitoring. Note: Drainage water from containers can be monitored. These indicate strength of salts and fertilizers still in the medium. Be sure compost has been wet for some time, to insure reading water from compost and not fresh irrigation water.
Other Uses
Space does not allow more in depth covering for all the uses of this meter. Briefly, others include — monitoring liquid fertilizer strength; checking for leaching of nitrogen in sawdust applications to rhododendron beds; being sure seedlings receive adequate feeding, but not too much; checking fertility of lawns, soil of pot plants, etc. In use, you will think of more applications.
Caution
— Do not use meter for testing batteries or other electronic applications. You will burn it out.
