JARS v36n3 - Unreduced Gametes in Azalea Hybrids: A Possible Breeding Method for Using Promising Azaleas of Low Fertility
Unreduced Gametes in Azalea Hybrids:
A Possible Breeding Method for Using Promising Azaleas of Low Fertility
1
Mark Widrlechner, Harold Pellett, and Peter Ascher
2
, Saint Paul, Minnesota
INTRODUCTION
Meiotic irregularities can result in the production of functional gametes with the full parental complement of chromosomes. Gametes having the same number of chromosomes as normal parental cells are called unreduced gametes. Unreduced gametes have an important role in plant evolution. Harlan and deWet (1975) reported numerous instances of polyploidy explainable by the functioning of unreduced gametes. For example, a tetraploid would result from the fusion of unreduced male and female diploid gametes and a triploid might be produced when unreduced pollen fertilizes a normal haploid ovule or when an unreduced ovule is fertilized by normal pollen.
Mature azalea pollen remains in groups of four (tetrads). Tetrads are the end product of normal meiosis; however, abnormalities can cause the formation of two large pollen grains (dyads) rather than tetrads. The male gametes produced by dyads are unreduced, each having twice as many chromosomes as normal gametes. Because of the gross difference in size between dyads and tetrads, it is simple to examine azalea pollen microscopically for the occurrence of unreduced gametes.
This report documents the existence of large pollen dyads in hybrid azalea, Rhododendron x kosterianum Schneider x R. prinophyllum (Small) Millais, F1 and F2 populations. Rhododendron x kosterianum is known as the mollis azalea and R. prinophyllum is the latin binomial for the pink-shell azalea, often called R. roseum . The F 1 populations have exceptional cold hardiness and have been released under the name Northern Lights (Pellett and deVos, 1978). They have been of little use, however, as breeding material because of low fertility. In addition, F 2 progenies often lack vigor and are less cold hardy than their F 1 , parents. Unreduced pollen from Northern Lights might overcome these problems since the parental genome would be transmitted more or less intact.
MATERIALS AND METHODS
Flowers were collected from field grown F
1
and F
2
plants of
R x kosterianum
x
prinophyllum
(Minnesota Landscape Arboreum accession numbers: 570494 D and F, 630002 D and N, 671039 B, E, and H, and 690331) populations at the Minnesota Landscape Arboretum, Chaska, Minnesota. Flowers were collected from about 2 weeks before anthesis to anthesis without any noticeable difference in pollen structure.
Pollen was spread onto a microscope slide by carefully crushing anthers and removing any large pieces of anther walls. At first, we suspended pollen in a dilute aniline blue solution, but later found that staining with a 0.5% aqueous solution of 3- (4,5-dimethylthiazolyl-2)--2,5-diphenyl tetrazolium bromide was more discriminating and had an added advantage of being a vital stain. This allowed us to calculate the proportion of live pollen grains (Hecker, 1963). When pollen is not stained it is inviable, but when the pollen is viable it stains bright magenta. The slides were then examined and photographed with a Zeiss RA microscope and a Nikon F2 camera. Tetrads and dyads were counted; and when the tetrazolium stain was used, presence of staining was noted.
RESULTS
Table 1 shows the proportion of tetrads and dyads found. Dyads were found in low frequencies, or not at all, in many of the samples. However, all four plants sampled from F
2
family 690331 produced detectable frequencies of unreduced pollen. Figure 1 shows a dyad from 690331 in comparison with smaller tetrads. As shown in Table 2, dyads generally stain, while tetrads often do not. When tetrads do stain, it is usual for only one or two grains of each to be stained. For sugar beet pollen, stainability is generally correlated with, but an overestimate of, ability to germinate (Hecker, 1963). We plan experiments to see if the same relationship holds for azalea pollen.
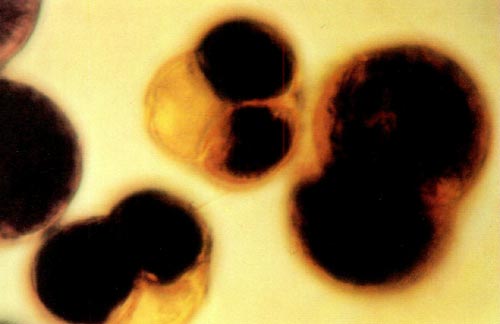
|
|
Fig. 1: This photomicrograph shows two tetrads and one dyad from
( R. x kosterianum x prinophyllum ) x ( R. x kosterianum x prinophyllum ) stained with a 0.5% aqueous solution of 3- (4,5-dimethylthiazolyl-2) -2, 5-diphenyl tetrazolium bromide. The dyad measures 55 by 80 microns and the two tetrads are approximately 50 microns in diameter. |
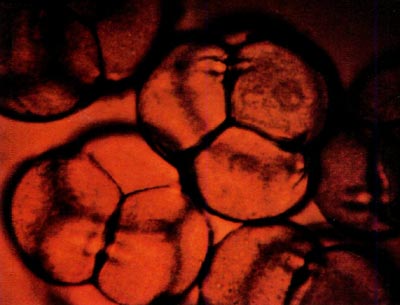
|
|
Fig. 2: This photomicrograph shows portions of five
tetrads from R. canadense x kosterianum stained with dilute aniline blue. The tetrads measure approximately 66 microns in diameter. |
DISCUSSION OF THE RESULTS
Li (1957) suggested that unreduced gametes might be found in azaleas; and Sax (1930) and Willingham (1976) each presented circumstantial evidence for diploid pollen. Sax counted chromosomes of a
R. calendulaceum
(Michx.) Torrey (2n= 52) x
R. occidentale
(Torrey & Gray) A. Gray (2n= 26) hybrid and Willingham cytologically examined
R. calendulaceum
x
R. alabamense
Rehd. (2n= 26) hybrids. They found most of the hybrids to be tetraploid, even though the pollen parent was diploid. They did not, however, rule out the possibility of contamination in the pollination process. In
Vaccinium
Camp (1945)found a complex series of hybrids that could be explained by assuming the union of unreduced gametes. Species of different ploidy levels exist in seven series of lepidote rhododendrons (Janaki Ammal, 1950) and in azalea Sections
Pentanthera
G. Don and
Rhodora
(L.) G. Don. It is probable these differences in ploidy arose because of the fusion of unreduced gametes.
Meiotic irregularities producing unreduced gametes are favored under certain circumstances. These include when: 1) the plant is stressed by environmental extremes; 2) under genetic control (Mutations can interfere with normal meiosis.); and in hybrids, 3) there is a lack of chromosome homology and poor pairing. A report by Veilleux and Lauer (1981) presents a comprehensive review of studies describing these conditions.
The azalea hybrids examined in this study were not grown under extreme conditions nor do we presently have reason to believe that they carry mutants of major genes controlling meiosis. However, R. x kosterianum x prinophyllum progenies may have an insufficient level of chromosome homology, to permit homeologous chromosomes to pair during meiosis. The low percentage of viable tetrads found in the hybrids lends support to this hypothesis. The situation is similar to one first described by Karpechenko (1927) for hybrids between Raphanus sativus L. and Brassica oleracea L. A cytological examination of meiosis of these azalea hybrids is in progress to confirm the hypothesis that poor pairing between homeologous chromosomes is interfering with meiotic cytokinesis (cellular division).
POSSIBLE APPLICATIONS
Interspecific crosses between azaleas (or rhododendrons) of the same ploidy level have often produced progeny of interest to the breeder; however, these hybrids may suffer decreased fertility. Following a breeding scheme proposed by Mendiburu et al. (1974) for potatoes, we would suggest using the hybrid as a pollen parent and using as a female a plant of ploidy level twice that of the male. For example, if one has a hybrid between two diploids of subseries Luteum, then one might use it as the pollen parent and a tetraploid, such as
R. canadense
(L.) Torrey (rhodora) or
R. calendulaceum
(flame azalea), as the female. Triploids are uncommon in
Rhododendron
(Janaki Ammal et al., 1950; Li, 1957) even in hybrids between diploids and tetraploids (Willingham, 1976). Thus one would expect progeny from this cross to contain tetraploid individuals resulting from the fusion of an unreduced gamete from the hybrid and a normal gamete from the tetraploid.
Depending on the type of meiotic irregularities involved in azalea dyad formation, unreduced gametes may or may not preserve a good deal of the heterozygosity of the hybrid. In potato, Mok and Peloquin (1975a) described three types of cytological abnormalities. Two types were observed to have a single premature cytokinesis. Gametes produced from these systems had increased homo-zygosity. In the other case, parallel spindles gave rise to gametes that preserved all heterozygous loci from the centromere to the first cross-over of each chromosome pair (if there was a cross-over at all) and half of the heterozygous loci from the first cross-over to the second. Unreduced gametes from parallel spindle plants produced more vigorous progeny than those from premature cytokinesis when crossed to tetraploids (Mok and Peloquin, 1975b).
If a system like the parallel spindle system is operating in azaleas, our crossing design should be a useful tool for increasing vigor and variety in polyploid species; for in tetraploid crops, such as potatoes (Mendiburu et al., 1974) and alfalfa (Busbice and Wilsie, 1966), allelic interactions and maximum heterozygosity have been found to contribute greatly to vigor. Even if a system like premature cytokinesis is present, there may be an increase in heterozygosity over that resulting from intraspecific crosses and an increase in variation beyond that now found in the parent of higher ploidy.
If it is true that tetraploid R. calendulaceum is involved in the parentage of Exbury and related cultivars, a statement questioned by Lee (1965), then it is likely that some of those cultivars are tetraploid and resulted unintentionally from the kind of crossing suggested above. Cultivars in those groups should be cytologically examined to explain what might otherwise be described as cross-incompatibility. There may be diploids and tetraploids within the same group.
Rhododendron canadense is a tetraploid with flowers of a mauve hue unusual for deciduous azaleas. Rehder and Wilson (1921) reported hybrids between R. canadense and members of ss. Luteum. These hybrids had larger flowers than R. canadense and one of them, R. x fraseri W. Watson, retained the unusual color. It is likely these hybrids resulted from the fertilization of R. canadense by an unreduced gamete. We have crossed R. x kosterianum with R. canadense and have progeny resembling R. x fraseri . They produce tetrads (Figure 2) suggesting that they are tetraploid and not triploid. One approach to developing large-flowered, deciduous azalea cultivars with flower color like R. canadense would be to select large-flowered, diploid cultivars of ss. Luteum for dyad production, and use those selections as pollen parents for crosses with R. canadense .
| Table 1 Proportion of Dyads and Tetrads in Hybrid Azalea Pollen | ||||
| Hybrids | Classes | |||
| % Tetrads | % Dyads | % Other* |
Total #
Counted |
|
| R. x kosterianum x prinophyllum | ||||
| Accession 570494D | 100.0 | 0.0 | 0.0 | 85 |
| 570494F | 99.3 | 0.5 | 0.2 | 1337 |
| 630002D | 99.8 | 0.2 | 0.0 | 576 |
| 630002N | 98.2 | 0.0 | 1.8 | 166 |
| R. prinophyllum x R. x kosterianum | ||||
| Accession 671039B | 99.6 | 0.2 | 0.2 | 484 |
| 671039E | 98.1 | 0.5 | 1.4 | 215 |
| 671039H | 98.8 | 0.2 | 1.0 | 567 |
|
(
R. x kosterianum
x
prinophyllum
) x
( R. x kosterianum x prinophyllum ) |
||||
| Accession 690331 #1 | 83.8 | 16.2 | 0.0 | 142 |
| 690331 #2 | 93.3 | 6.7 | 0.0 | 223 |
| 690331 #3 | 79.1 | 20.9 | 0.0 | 163 |
| 690331 #4 | 91.6 | 7.2 | 1.2 | 83 |
| *Other includes individual or broken grains, triads, and undetermined groupings. | ||||
| Table 2 Per Cent Hybrid Azalea Pollen Stained with Tetrazolium Bromide | ||
| Hybrids | % Stained | Total # Counted |
| R. x kosterianum x prinophyllum | ||
| Accession 570494F Tetrads | 27 | 154 |
| Dyads | 100 | 6 |
| R. prinophyllum x R. x kosterianum | ||
| Accession 671039E Tetrads | 13 | 211 |
| Dyads | 100 | 1 |
|
(
R. x kosterianum
x
prinophyllum
) x
( R. x kosterianum x prinophyllum ) |
||
| Accession 690331 #1 Tetrads | 58 | 119 |
| Dyads | 100 | 23 |
| 690331 #2 Tetrads | 54 | 208 |
| Dyads | 100 | 15 |
| 690331 #3 Tetrads | 23 | 129 |
| Dyads | 97 | 34 |
SUMMARY
The existence of unreduced gametes in azaleas has been confirmed. A general breeding system to take advantage of this phenomenon is described and specific crosses are suggested.
ACKNOWLEDGEMENTS
We sincerely thank Doctors Florian Lauer, David Davis, Sharon Desborough, and Charles Burnham for their helpful suggestions. Also the laboratory assistance of Sue Fuhrman is greatly appreciated.
Literature Cited
Busbice, T.H. and C.P. Wilsie. 1966. Inbreeding depression and heterosis in autotetraploids with application to
Medicago sativa
L. Euphytica 15: 52-67.
Camp, W.H. 1945. The North American blueberries with notes on other groups of
Vacciniaceae
. Brittania 5: 203-275.
Harlan, J.R. and J.M.J. deWet. 1975. On Ö. Winge and a prayer: the origins of polyploidy. Bot. Rev. 41: 361-390.
Hecker, RJ. 1 963. Use of tetrazolium salts in determining viability of sugar beet pollen. J. Am. Soc. Sugar Beet Technol. 12: 521-528.
Janaki Ammal, E.K. 1950. Polyploidy in the genus
Rhododendron
. Rhod. Yrbk. Roy. Hort. Soc. 5: 92-98.
___ , ___ , I.C. Enoch, and M. Bridgewater. 1950. Chromosome numbers in species of
Rhododendron
. Rhod. Yrbk. Roy. Hort. Soc. 5: 78-91.
Karpechenko, G.D. 1927. The production of polyploid gametes in hybrids. Hereditas 9: 349-368.
Lee, F.P. 1965. The Azalea Book, 2nd ed. Princeton, NJ: D. Van Nostrand.
Li, H.L. 1957. Chromosome studies in the azaleas of eastern North America Amer. J. Bot. 44: 8-14.
Mendiburu, A.O., S.J. Peloquin, and D.W.S. Mok. 1974. Potato breeding with haploids and 2n gametes. Proc. Int'l Haploid Symp. on Higher Plants, pp. 249-258
Mok, D.W.S. and S.J. Peloquin. 1975a. Three mechanisms of 2n pollen formation in diploid potatoes. Can. J. Genet. Cytol. 17: 217-225.
___,___and___,___1975b. Breeding value of 2n pollen (diplandroids) in tetraploid x diploid crosses in potatoes. Theoret. Appl. Genet. 46: 307-314.
Pellett, H. and F. deVos. 1978. Northern Lights, new winter hardy azalea hybrids. Univ. of Minn. Agric. Expt. Sta. Misc. Report 155.
Sax, K. 1930. Chromosome stability in the genus
Rhododendron
. Amer. J. Bot. 17: 247-251
Veilleux, R.E. and F.I. Lauer. 1981. Variation for 2n pollen production in clones of
Solanum phureja
Juz. and Buk. Theoret. Appl Genet. 59: 95-100.
Willingham, F.F., Jr. 1976. Variation and phenological forms in
Rhododendron calendulaceum
(Michx.) Torrey (
Ericaceae
). Castanea 41: 215-223.
Wilson, E.H. and A. Rehder. 1921. A Monograph of Azaleas.
Rhododendron
subgenus
Anthodendron
. Cambridge, MA: Arnold Arboretum.
1
Scientific Journal Series Paper No. 11,842, Minnesota Agricultural Experiment Station.
2
Research Assistant and Professors, respectively, Department of Horticultural Science & Landscape Architecture, University of Minnesota, St. Paul, MN
