JARS v37n4 - Light Colour and Indol-3yl-butyric Acid Soak Time Effects on Rhododendron 'Blue Tit' and R. 'Dora Amateis'
Light Colour and Indol-3yl-butyric Acid Soak Time Effects on
Rhododendron 'Blue Tit' and R. 'Dora Amateis'
Lisa and Nicholas Yarmoshuk
Editor's Note: All work reported here was performed by Lisa from March 1979 to April 1982. The second author prepared this paper for publication and provided design and statistical advice. Lisa represented the Niagara Regional Science and Engineering Fair with this project at the 32nd International Science Fair, May 1982 at Houston, Texas and was awarded the United States Army Gold Medal, the United States Department of Agriculture First Prize and The General Motors Second Place Grand Prize in Botany. She worked at the USDA Florist and Nursery Crops Laboratory located at the Beltsville Agricultural Research Center, Beltsville, M.D., in July and August 1982 and is currently a grade twelve student at Sir Winston Churchill Secondary School, St. Catharines, Ontario.
Hartman and Kester's (2), Loach's (4), Wells' (9) and our 1970-1981 searches of the research literature identified twenty-two important factors affecting root development on stem cuttings. Light quality (intensity, period and colour) is always implied as an important element, yet references are brief, vague and contradictory. Loach (4) suggests low light levels and 100 w tungsten filament lamps set at two metres apart and one metre above the bench, providing 18 hour days, as a desirable light quality. Wells (9) and Leach (3) encourage as much light as possible but do not specify daylength. Schram (6) advocates 16 hour days with two cool-white 48" fluorescent tubes, one foot above the cuttings. The unstated commonality in Loach's and Schram's assertions is that both light sources are rich in red and far-red frequencies (>620nm). Our review unearthed a few reports on the role of light frequencies on root development of seedlings, but no study of their effects on rooting stem cuttings. Since the role of phytochrome, a plant protein sensitive to light at 660nm and 735nm, is well known (2,7,8) as a trigger and inhibitor of seed germination, it occurred to the senior author that it may be fruitful to explore the potential contribution of red light to root development on stem cuttings.
The importance of Indol-3yl-butyric Acid (IBA) in rooting is well documented (2,3). Although dry-dip application of IBA/talc formulations is practical for mass production and for easy to root cultivars, it is difficult to control accurately amount of powder applied to a wound. Dipping into highly concentrated liquid IBA solutions is subject to timing inaccuracies and unreliable absorption rates. Long soak-times (3) present difficulty in identifying the best soak-time/ IBA concentration combination.
This report describes four controlled studies of the differential responses to blue (420-520nm) and red (>620nm) light rays of stem cuttings of two lepidotes and simultaneously tests the effects on rooting of one IBA concentration at various soak times.
Method
R. 'Blue Tit' and R. 'Dora Amateis' were used in a replicated 3 x 7 fixed factor design (Figure 2) with 21 conditions per cultivar. Ten cuttings are the minimum appropriate for experimental study. Since a plastochron index (1) is not available for rhododendrons it is important to minimize, through design, the potential variability in stem cutting properties. We used one mother plant for 'Blue Tit' and two for 'Dora Amateis' to supply the cuttings. The latter two plants were originally propagated from the same mother plant. These cultivars' growth habits and shoot properties provided sufficient cuttings to meet cell requirements. Of 22 factors which may affect rooting, A1 to A5 listed below are constants for each mother plant. The other factors may introduce error.
Procedures described here were also performed with cuttings of a florist's azalea, Juniper 'Blue Acre', Cotoneaster, and in a more simple design with R. smirnowii and R. smirnowii x 'Lady Bessborough'. Space precludes reporting full details for this additional work, but the results will be integrated with those of the lepidote studies.
Stock Plant Characteristics and Stem Cutting Treatments
A. Stock Plant Characteristics
1.
Nutrition
. No fertilizer used during two years prior to taking cuttings.
2.
Growth Medium
. R. 'Dora Amateis' grown in Port Colborne Peat amended with sand, pine needles and sheep manure. R. 'Blue Tit' grown in sandy loam amended with peat moss.
3.
Date Taken
. 4 pm, October 16, 1980 and kept in cold storage to 9 am, October 17, 1980. Repeated October 21, 1981.
4.
Age
. R. 'Blue Tit', 11 yrs. R. 'Dora Amateis', 6 yrs.
5.
Photoperiod
. Natural days.
6.
Light Intensity
. R. 'Blue Tit' 80% shade HR10 woodlot, Vineland. 'Dora Amateis', 60% shade, 57 Highland Ave., St. Catharines, Ont.
7.
Stem Illumination
. Exposed to light according to position on plant. Cuttings from entire plant periphery assigned randomly to experimental conditions.
B. Stem Cutting Treatments
1.
Stem Length
. 2 inches.
2.
Stem Diameter
. Wide variation. No plastochron index; cutting assigned at random to conditions.
3.
Leaf Number
. R. 'Blue Tit', 6-9, R. 'Dora Amateis', 3-4.
4.
Disbudding
. R. 'Dora Amateis' disbudded 2 months prior to taking cuttings. 'Blue Tit' disbudded prior to item 6.
5.
Wounding
. No wounding other than during leaf stripping.
6.
Disease Control
. All cuttings washed in physan, 1 tbsp/litre water prior to IBA immersion. All equipment washed in Clorox.
7.
Air/Soil Temperature
. Night / day extremes, 18°C to 26°C, maintained with fans exterior to "Wardian Cases". Heat accumulation under PLEXIGLASS is acute problem compared to use of 6 mil plastic sheets. Temperatures between light conditions constant within thermometer accuracy. Bottom heat provided by cable and tiered design.
8.
Hormone Treatment
All cuttings in each condition soaked in beaker with 100 ml of 150 ppm IBA solution.
9.
Soak Temperature
. 15° C
10.
Light Conditions During Soaking
. Soaked in total darkness in three different rooms according to light colour assignment. Safe-lights used when working in and entering rooms. At no time was a soaked or planted cutting exposed to a light colour other than that to which it had been assigned.
11.
Rooting Medium
. 2 parts sphagnum moss 1 part perlite; very lightly tamped into pot. One cutting assigned to each 2 inch plastic pot.
12.
Tissue Turgidity
. 100% humidity used twice weekly; sprayed with Captan/Benlate WP solution.
13.
Photoperiod
. 18 hour days, timed.
14.
Photointensity
. Two new f72T12/GRO/VHO/WS Sylvania fluorescent tubes on 21 cm centers, 29 cms above cutting surface, per light condition. 2" pots in holding trays moved laterally daily and turned front to back to balance light exposure at tube ends.
WIDE SPECTRUM GROLUX TUBES were placed over "Wardian Cases" constructed from 4'x 8' x ¼" sheets of Rohm and Haas #2423 and #2424 PLEXIGLASS, which acted as filters, admitting only the experimentally desired light rays. The "cases" with light tight joints fit into light traps. Clear PLEXIGLASS was used as a control. Filter spectrophotometer curves and fluorescent tube light emission characteristics are shown in Figure 1. Kodak gelatin filters would be more frequency specific but their cost was prohibitive.
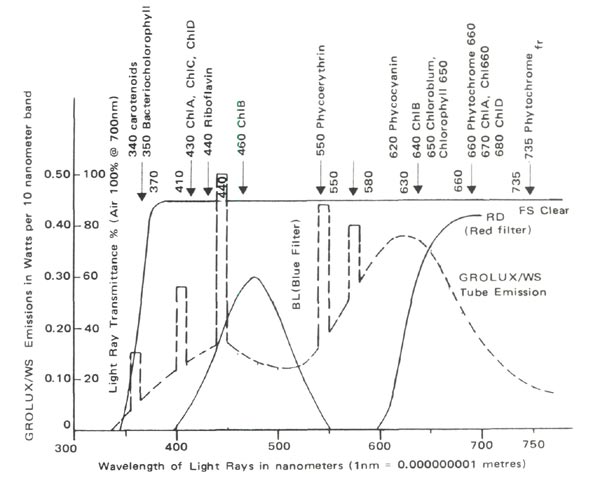
|
|---|
Figure 1 |
Five soak times (6, 12, 18, 24, 30 hours), a no-soak and an ethanol/ water 18 hour soak condition were used in all trials. This is a test of Leach's 18 hour optimum soak time for low concentration IBA solutions. IBA crystals were dissolved in ethanol and diluted in water to obtain a 150 ppm solution. The no-soak and ethanol/water conditions are controls to test for the effects of water and ethanol. (3) page 319.
Rooting percent, number of root initials and projected root area are the dependent variables. Projected root area was assessed using a Rhizometer constructed after Morrison and Armson (5). Number of root initials obtained by actual count is a good indicator of rooting activity; this is useful only on plants with thick roots and is impractical for fine root haired plants such as rhododendrons.
|
Figure 2
Rooting Rates for R. 'Dora Amateis' (DA), R. 'Blue Tit' (BT) & Juniper 'Blue Acre' (JBA) Rooted in 1980/81 (t 1 ) and/or 1981/82 (t 2 ) |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Indol-3yl-butyric Acid (150 ppm) Soak Time (hours) |
|||||||||||||||||||||
Light color |
Variety |
Ethanol/H 2 O 18 hours |
No Soak |
6 |
12 |
18 |
24 |
30 |
Overall |
||||||||||||
t 1 |
t 2 |
t 1 |
t 2 |
t 1 |
t 2 |
t 1 |
t 2 |
t 1 |
t 2 |
t 1 |
t 2 |
t 1 |
t 2 |
t 1 |
t 2 |
||||||
CLEAR (>320 nm) |
DA |
8/10 |
8/10 |
3/10 |
3/10 |
2/10 |
9/10 |
7/10 |
8/10 |
6/10 |
8/10 |
9/10 |
10/10 |
10/10 |
7/10 |
62% |
75% |
||||
BT |
11/18 |
8/10 |
10/18 |
8/10 |
14/18 |
9/10 |
14/18 |
9/10 |
13/18 |
5/10 |
9/18 |
7/10 |
13/18 |
8/10 |
67% |
68% |
|||||
JBA |
-- |
9/10 |
-- |
0/10 |
-- |
1/10 |
-- |
2/10 |
-- |
8/10 |
-- |
10/10 |
-- |
9/10 |
-- |
50% |
|||||
BLUE (420-520 nm) |
DA |
2/10 |
0/10 |
2/10 |
0/10 |
2/10 |
0/10 |
4/10 |
0/10 |
1/10 |
0/10 |
1/10 |
0/10 |
1/10 |
0/10 |
18% |
0 |
||||
BT |
0/10 |
0/10 |
0/10 |
/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0 |
0 |
|||||
JBA |
-- |
0/10 |
-- |
0/10 |
-- |
0/10 |
-- |
0/10 |
-- |
0/10 |
-- |
0/10 |
-- |
0/10 |
0 |
0 |
|||||
RED (>620 nm) |
DA |
10/10 |
4/10 |
10/10 |
7/10 |
10/10 |
10/10 |
10/10 |
8/10 |
10/10 |
8/10 |
10/10 |
10/10 |
10/10 |
10/10 |
100% |
91% |
||||
BT |
11/18 |
7/10 |
13/10 |
4/10 |
9/18 |
7/10 |
14/18 |
3/10 |
11/18 |
6/10 |
13/18 |
10/10 |
13/18 |
6/10 |
67% |
60% |
|||||
JBA |
-- |
0/10 |
-- |
1/10 |
-- |
0/10 |
-- |
0/10 |
-- |
10/10 |
-- |
10/10 |
-- |
10/10 |
-- |
51% |
|||||
Results Discussion and Conclusions
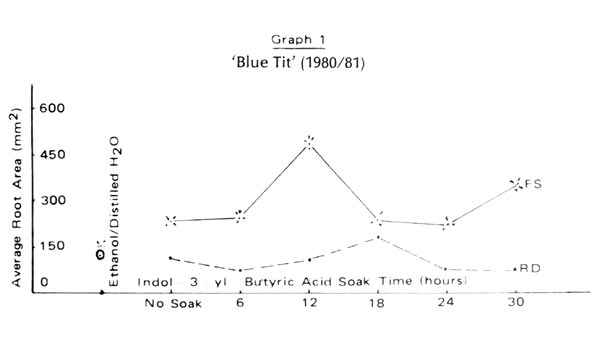
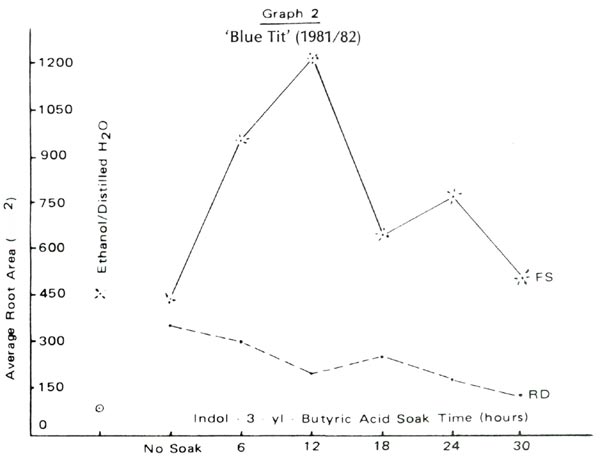
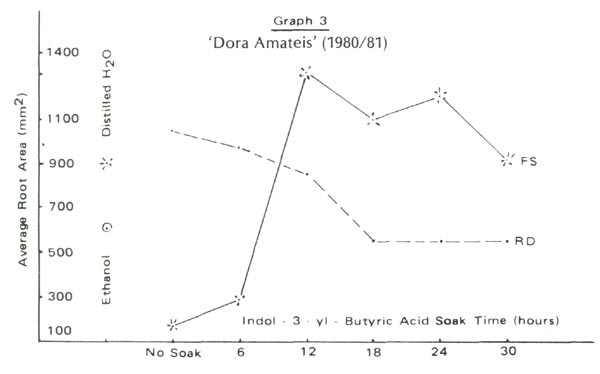
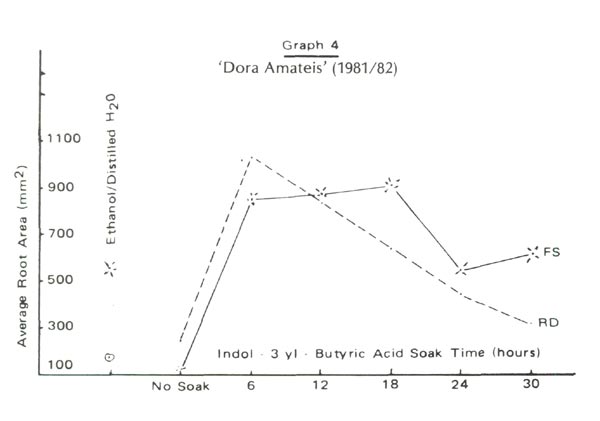
Figure 2 and Graphs 1 to 4 show rooting percentages and average root areas after 98 days for each experimental condition. Rooting was not supported by blue light rays (BL) but was well supported by red (RD) and full spectrum (FS) light. Rooting rates for R. 'Blue Tit' were similar under RD and FS. For 'Dora Amateis' rooting rates in the RD condition were greater than under FS. Total lack of response to BL and growth under RD and CL was found for a florist's azalea, Juniper Blue Acre and Cotoneaster. Although we cannot say that the phytochrome is involved in root development, it is clear that the spectrum >620nm is active in root formation and development, while the 420-520nm range is not.
|
|---|
|
|---|
|
|---|
|
|---|
Is non rooting in the BL condition due to the fact that the quantity of light (the number of photons in the photosynthetically bands) is not identical in each treatment? In the three light treatments, the light quantities were in the ratio 1:2:7 for blue, red and full spectrum treatments respectively. Rooting under BL was unsuccessful in an earlier experiment employing July full sun [with appropriate ventilation]. Furthermore, statistical analysis of the data of Graphs 1-4 do not reveal the consistent significant differences between RD and FS root areas one would expect if a light quantity effect was present.
Using rooting percent alone we could suggest that rooting under RD alone is superior to rooting under FS. But graphs 1 to 4 show GREATER ROOT AREA development under FS than under RD. We doubt that this is an intensity effect because the attenuation of the red waves is only 5%. We speculate that root initiation is encouraged by RD and once started, the roots develop more rapidly under the influence of other parts of the spectrum. Testing this idea requires more discriminating filters to identify the involved frequencies and a willingness to count root initials. The second step was followed with Juniper Blue Acre. Paradoxically root initiation was greater under FS, but root area was greater under RD. This suggests a light frequency, in addition to RD which supports root initiation and other frequencies which do not support root development. Clearly, rooting percentages alone do not provide sufficient information to allow more complete understanding of rooting behavior.
Under perfect experimental conditions each cutting within a cell would develop the same root area as any other cutting in that cell. Individual differences cause a wide range of root areas to be observed within a condition. Root areas plotted in Graphs 1 to 4 are averages computed from the wide range. The smaller the root area variation the lower is the potential error. Under RD the variance in root area is one-half that under FS for each of the four lepidote trials . This is also true for each trial with the florist's azalea, Cotoneaster and Juniper 'Blue Acre'. We suggest that explanation for this may lie in differential contributions of certain parts of the spectrum to root initiation and development.
R. smirnowii and R. smirnowii x 'Lady Bessborough' were used in a 2 x 2 design to test the effects of RD and FS on rooting. After 99 days all cuttings under FS were rooted, 7/20 cuttings under RD were rooted. R. smirnowii had heavy root balls, small but clear trace roots were on the latter. Apparently R. smirnowii x 'Lady Bessborough' requires more time to form roots than R. smirnowii . Very heavy callusing was observed on 6/20 rooted stems under FS, superficial callusing appeared on 7/20 cuttings under RD. The remainder were free of callus. The role of light rays on callus development is worthy of detailed study.
For the two lepidotes a 12 to 18 hour soak time appears optimum. Rooting percent would suggest nodifferences in cell performance yet the graphs of root area variation show variations among soak times. 'Dora Amateis' rooted equally well in trials 1 and 2, but root areas in trial 2 were lower than in trial 1. While the rooting percentages for 'Blue Tit' were consistent for the two trials the root areas in trial 2 were greater than in trial 1. This could be a seasonal or stem growth effect rather than any other effect because the relative shapes of the curves for the two trials are very similar. The importance of root areas as compared to rooting percentages is further exhibited by comparing rooting percentages and root areas for the controls and thirty hour soaks under the RD and FS conditions.
The effect of soak time is dramatically exhibited with the Junipers. In figure 2 no rooting is exhibited at 150ppm until the 18 hour soak condition. At that condition rooting goes from 0 to 100% under both RD and FS.
These data support the use of tungsten filament lamps and/or simple cool-white fluorescent tubes for rooting cuttings of at least two lepidotes, two elepidotes and Juniper Blue Acre. Low light levels are adequate for rooting. 12-18 hour soak times are effective for these cultivars with IBA 150ppm solutions. The high humidity, maintained at moderate temperatures, in a very loose lightly moistened medium allowing high oxygen levels probably provided ideal rooting environment.
Bibliography
1. Coleman, W.K. and Greyson, R.I. The growth and development of the leaf in tomato (Lycopersicon esculentum). 1. The plastochron index, a suitable basis for description.
Canadian Journal of Botany
. Volume 54.
2. Hartman, H.T. and Kester, D.E. Plant Propagation: Principles and Practice . Third Edition. Prentice Hall. 1975.
3. Leach, D.G. Rhododendrons of the World . Charles Scribner's Sons. 1961.
4. Loach, K. Rhododendrons from stem cuttings. Rhododendrons 1977: With Magnolias and Camellias. The Royal Horticultural Society. Vincent Square, London, England. 1977.
5. Morrison, I.K. and Armson, K.A. The rhizometer - a device for measuring roots of tree seedlings. Forestry Chronicle , Volume 44, No. 5, 1968.
6. Schram, B. Private communications. 1983.
7. Smith, H. Phytochrome and Photomorphogenesis . McGraw-Hill. Maidenhead Berkshire, England. 1975.
8. Veen, R. vander and Meijer, G. Light and Plant Growth . The Macmillan Company. 1962.
9. Wells, J.S. The propagation of hybrid rhododendrons from stem cuttings: An historical review. Journal of the American Rhododendron Society , Volume 36, Number 4, Fall, 1982.
10. Winer, B.J. Statistical Principles in Experimental Design 3rd Edition. McGraw-Hill. 1979.
Acknowledgments
Lisa acknowledges the kind support of T.H. Elliot, Vice-President, Rhom and Haas Canada; T.S. Dugard, GTE Sylvania Canada; A.W. Smith, Horticultural Research Institute of Ontario, Vineland; C. Graham, Propagator, Royal Botanical Gardens, Hamilton; who provided the required materials and stem cuttings without which this project would have been impossible.

