JARS v38n4 - Root Temperature Effects on Cold Acclimation of an Evergreen Azalea
Root Temperature Effects on Cold Acclimation of an Evergreen Azalea
Leslie A. Alexander and John R. Havis
Department of Plant and Soil Sciences
University of Massachusetts, Amherst, MA
Reprinted from HortScience 15(1):90-91. 1980
Abstract
Warm root environments interfered with cold acclimation in roots and lower stems sections of Rhododendron cv. Springtime.
Cold acclimation in woody plants is a physiological process brought about by a complex of environmental factors. It is generally agreed that acclimation proceeds in 2 phases. The progressive shortening of days in later summer, perceived by the leaves, is the stimulus for the first phase (5, 6, 7, 11). The second stage is mediated by low temperature (4, 7). Through the integration of these stimuli, the plant becomes acclimated.
Not all plants acclimate at the same time, and certain hardy woody species are injured by early autumn freezes in northern climates due to their tendency to acclimate slowly (3, 13). This problem is associated with plants native to areas of milder climates (13). The nature of cold injury problems associated with evergreen azaleas in the north suggest that these plants may be slow acclimators. Under the same conditions, the lower stem of an evergreen azalea acclimated more slowly than a deciduous azalea (1). Creech and Hawley (2) found that 10 cm mulch around azaleas in the fall kept the soil warm, delayed the development of fall color and increased the amount of winter injury, including bark splitting. Sakai (9) studying a variety of woody plants, found that the basal sections of the stems were less hardy than the upper parts of the plants.
The following study was carried out to determine the environmental effects of root temperature on cold acclimation of branches, lower stem and roots of an evergreen azalea. Beginning September 1, 1977, 1 year old plants of the evergreen azalea 'Springtime', a kaempferi hybrid, were placed in 15 cm diameter watertight plastic azalea pots, in a 1 peat: 1 sand mix (by volume), with 2 cm insulation covering the media surface. The roots of 18 plants were kept warm in a water bath at 23° ±2°C the first half of September, 20° ±2° the second half of September, and 18° ±2° until termination of the experiment December 20, 1977. Another 18 plants were maintained with cool roots of 7° ±1° for the first 2 weeks of September, 4° ±1° for the next 2 weeks, and 2° ±1° for the remainder of the experiment. It is possible that the temperatures of the lower stems, which were not monitored, were influenced by the root media temperatures. Air temperatures were similar at the lower stem levels of the two treatments. The plants were kept out of doors until October 18, when they were placed in a structure covered with clear polyethylene where the air was kept above freezing.
Branch, stem, and root were sampled and frozen as described previously (1) at 3 week intervals starting September 1, and continuing through December. Injury was determined using the modified ninhydrin test (14), using a release of 35% of the total ninhydrin-reactive compounds to represent the killing temperature (1). The killing temperature was confirmed by freezing extra tissue in separate tubes and inspecting for browning.
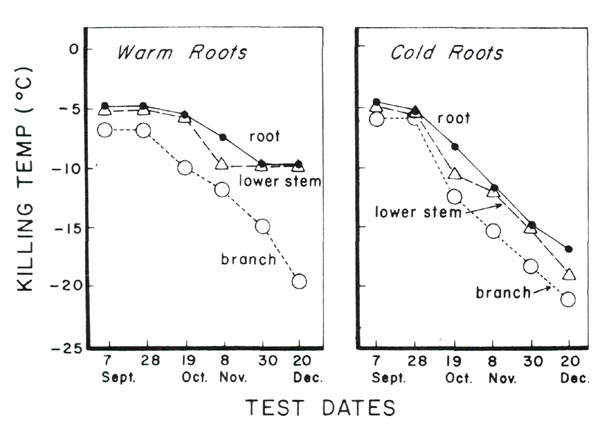
|
|
Fig. 1. Cold acclimation of branch, lower stem and root of 'Springtime'
azalea plants subjected to warm and cold root environments. |
All parts of the plants in the cold root environment acclimated faster and to greater degree than the plants with warm roots (Fig. 1). The lower stems and roots exhibited the greatest differences in acclimation. On the final test date, December 20, the roots and lower stems of plants in the cold environment were 7°C hardier than those in the warm environment.
In addition to variations in cold hardiness of plant parts, visual differences in the foliage were noted. The azaleas in the cold root environment attained full fall color by the end of October but plants in the warm root environment did not attain full fall color during the course of the experiment. Fall color may be an indicator of hardiness in this azalea species. Van Huystee et al. (12) observed that red stem pigmentation in red osier dogwood appeared to coincide with the development of cold resistance of the bark. Parker (8) reported that the increase in cold hardiness in Hedera helix was closely related in time to a sharp increase in total sugar and anthocyanin content. However, Steponkus and Lanphear (10) found no direct causal relationship between anthocyanin content and the development of cold hardiness.
All parts of the cold root plants acclimated steadily during the study. The warm root treatment produced a pattern of cold acclimation in lower stems and roots quite different from the cold root treatment. In September and October, these tissues in the warm root plants were less hardy but acclimated to some extent, reaching killing temp of 10°C November 8. However, the warm root treatment appeared to prevent any further acclimation of lower stems and roots during November and December, even though the upper branches continued to acclimate during this period.
One might speculate that lower stems and roots of evergreen azaleas go through two stages of acclimation, the latter of which require cool roots - at least cooler than the minimum 16°C to which the warm root plants were exposed in this experiment.
It is obvious that cold acclimation of all plant parts was greater in plants with cold roots than in plants with warm roots. A particularly interesting result was that the lower stems maintained essentially the same hardiness as the roots throughout the test period in plants of both treatments. These latter results agree with acclimation studies of another cultivar of evergreen azalea, 'Mother's Day' (1).
Literature Cited
1. Alexander, L.A. and J.R. Havis. 1980. Cold acclimation of plant parts in an evergreen and a deciduous azalea.
HortScience
15:89-90.
2. Creech, J.L and W. Hawley. 1960. Effects of mulching on growth and winter injury on evergreen azaleas.
J. Amer. Soc. Hort. Sci.
75:650-657.
3. Flint, H.L. 1966. Seasonal hardening in trees and shrubs.
Arnoldia
20:57-60.
4. Fuchigami, L.H., C.J. Weiser, and D.R. Evert. 1971. Induction of cold acclimation in
Cornus stolonifera
Michx.
Plant Physiol.
47:98-103.
5._________D.R. Evert, and C.J. Weiser. 1971. A translocatable cold hardiness promoter.
Plant Physiol.
47:164-167.
6. Hurst, C., T.C. Hall, and C.J. Weiser. 1967. Reception of the light stimulus for cold acclimation in
Cornus stolonifera
Michx.
HortScience
2:164-166.
7. Irving, R.M. and F.O. Lanphear. 1967. Environmental control of cold hardiness in woody plants.
Plant Physiol.
42:1191-1196.
8. Parker, J. 1962. Relationships among cold hardiness, water soluble protein, anthocyanins and free sugars in
Hedera helix
L.
Plant Physiol.
37:809-813.
9. Sakai, A. 1968. Frost damage on basal stems in young trees.
Contr. Inst. Low Temp. Sci.
B 15:1-14.
10. Steponkus, P.L. and F.O. Lanphear. 1969. The relationship of anthocyanin content to cold hardiness of
Hedera helix
.
HortScience
4:55-56.
11. _________ and _________ 1967. Light stimulation of cold acclimation: production of a translocatable promoter.
Plant Physiol.
42:1673-1679.
12. Van Huystee, R.B., C.J. Weiser, and P.H. Li. 1967. Cold acclimation in
Cornus stolonifera
under natural and controlled photoperiod and temperature.
Bet. Gaz.
128:200-205.
13. Weiser, C.J. 1970. Cold resistance and injury in woody plants.
Science
169:1269-1278.
14. Wiest, S.C., G.L. Good, and P.L Steponkus. 1976. Evaluation of root viability following freezing by the release of ninhydrin-reactive compounds.
HortScience
11:197-199.
