JARS v39n4 - Desiccation Injury and Direct Freezing Injury to Evergreen Azaleas: A Comparison of Cultivars
Desiccation Injury and Direct Freezing Injury to Evergreen Azaleas:
A Comparison of Cultivars
Peter M. Rosen, George L. Good, and Peter L. Stepponkus
Department of Floriculture and Ornamental Horticulture
Cornell University, Ithaca, NY
Reprinted from Journal American Horticultural Science
Abstract
Four cultivars of kurume azalea (
Rhododendron
sp.), 'Hershey's Red', 'Snow', 'Coral Bells' and 'Hino Crimson' reported to have different susceptibilities to winter injury in the nursery were compared for their sensitivities to direct freezing injury (both to roots and leaves) and desiccation injury under controlled conditions. Sensitivity to root freezing injury was inversely correlated with winter injury. A positive association between leaf hardiness to freezing injury and resistance to winter injury was found only in 'Hino Crimson'. Susceptibility to winter injury was most closely associated with desiccation, as indicated by the minimum water potentials under conditions of frozen soil and high evaporative demand.
Winter injury is a major problem associated with the production of containerized nursery stock in the northeastern United States. Broad-leaved kurume azaleas are particularly sensitive to injury during overwintering, even in unheated, polyethylene-covered, Quonset-type structures. Observations by commercial nurserymen on Long Island, N.Y., indicated that the cultivars 'Snow' (SN), 'Hino Crimson' (HC), 'Coral Bells' (CB), and 'Hershey's Red' (HR) have significantly different susceptibilities to winter injury. SN is the most susceptible, HR is the most resistant, and CB and HC are intermediate.
Visible injury to leaves, which occurs during the winter or early spring in these cultivars, has been attributed to 3 major factors: desiccation injury, direct freezing injury to roots, and direct freezing injury to leaves. Desiccation injury can occur as a result of high evaporative demand in conjunction with soil freezing in the root zone (4). Freezing injury to roots can occur as the result of low root zone temperatures in the containers exposed above the ground surface (3, 5, 7). Freezing injury to leaves may also be a factor, as identified by Devenport (1).
Since all 3 types of stress can occur in azalea plants over-wintered in containers in polyethylene structures, it is difficult to separate the role of each in explaining the differences in susceptibility to winter injury between the 4 cultivars. The objective of this research was to elucidate the role of each type of stress in accounting for varietal susceptibilities to winter injury.
Materials and Methods
Two-year-old plants grown under natural conditions in 3.8 liter (1 gallon) containers, were brought from Long Island to Ithaca, N.Y., in late November, and maintained outdoors until late December to assure a maximum degree of cold acclimation. They were then brought into a controlled-environment chamber at 2°C, with 12 µE m
-2
sec
-1
photosynthetically active radiation (PAR) and photo period of 9 hr.
Whole plants were cooled at a rate of 2.5°C/hr in the dark to the desired temperatures; warming was also at 2.5°/hr. The elapsed time for cooling, maintenance at the desired temperature, and warming was 16 to 23 hr. Leaf injury was the visual estimate of percent of necrotic leaf area. Freezing injury to excised leaf discs also cooled and warmed at 2.5°/hr was assayed by the triphenyl tetrazolium chloride (TTC) method (6) and freezing injury to excised roots was assayed by the ninhydrin method (8).
Transpiration rates were determined by whole plant lysimetry, with leaf area determined subsequently by the ratio of dry weight for a known leaf area and the dry weight of total plant leaf area. Transpiration rates in frozen soil were measured on plants for which the pots (not including plant tops) had been frozen to -5°C for the previous 16 hr. Stomatal resistance (r s ) was measured with a diffusion porometer (Lamda Instruments, Model LI65) on the lower surface of fully expanded leaves. Stem water potentials, ψ, were measured with a pressure bomb (PMS Instruments, Model 1000) on excised shoots.
Results
Direct Freezing Injury
.
To determine the effect of low temperature in the absence of desiccation stress, whole plants were frozen and thawed in the dark, and leaf injury was scored 2 days or 3 months after freezing (Table 1). When symptoms were observed 2 days after freezing to -14°C, CB had the greatest degree of injury, SN was intermediate, and HR and HC had a relatively low degree of injury. After 3 months of storage in the 2° environment, leaf injury in those plants showed a different ordering among cultivars, with a high degree of injury in HR and CB, and less injury in HC and SN.
Although the values for the percent of necrotic leaf area (Table 1), which was 0% in unfrozen controls, represent percentages of different total leaf area at the 2 assay times, the difference between cultivars in the increment during storage does not represent a difference in the extent of leaf drop. Some leaf abscission did occur in those plants, but there was no significant differences between cultivars in the extent of leaf drop (data not shown). The increment in leaf injury during those 3 months was greatest in HR, intermediate in CB and HC, and lowest in SN (Table 1).
| Table 1. Leaf injury to 4 cultivars of azalea after freezing and thawing of whole plants, in the dark, to -14°C. | ||||
| Leaf area necrotic (%) | ||||
| Treatment | Snow | Coral Bells | Hino Crimson | Hershey's Red |
| 2 days after freezing | 41 ab | 58 a | 21 b | 18 b |
| 3 months after freezing | 34 b | 84 a | 44 b | 79 a |
| N = 4, mean separation within rows by Duncan's multiple range test (5% level) | ||||
The extent of leaf injury present 2 days after freezing was indicative of direct freezing injury to plant tops, because indirect leaf injury resulting from freezing damage to the roots was not yet evident at that time. Determinations of leaf injury by the TTC method (Fig. 1) after freezing of excised leaf discs suggested a similar ranking, but the killing temperatures at 50% absorbance were not statistically different. Thus, at -14°C, only the visual assay showed the greatest degree of leaf injury in CB, an intermediate degree of leaf injury in SN, and lesser degrees of leaf injury in HC and HR.
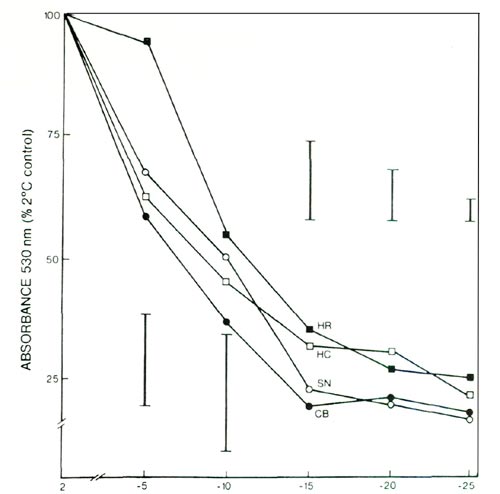
|
|
Fig. 1. Triphenyl tetrazolium chloride test of freezing injury to
leaves of 4 azalea cultivars; see text for cultivar abbreviation. Vertical bars indicate maximum SE at a given temperature, n = 4. |
The extent of leaf injury 3 months after freezing represented a combination of direct freezing injury to leaves and indirect injury resulting from freezing damage to roots. Comparing cultivars, the increment in injury between 2 days and 3 months was indicative of the relative degree of root injury during the original freezing treatment. Determination of root injury by the ninhydrin method (Fig. 2) after freezing of excised roots, gave similar results to the 3-month treatment. At -14°C, both methods showed the greatest degree of root injury in HR and CB, intermediate degrees of root injury in HC, and the least degree of root injury in SN. (Note that the ordinates for the graphic representations of the TTC and the ninhydrin tests are inverted relative to one another. In the TTC test, controls are unfrozen plants, whereas in the ninhydrin test the controls are boiled to remove all the ninhydrin-reactive compounds). The fact that the increment in visible injury for CB is somewhat less than might be expected if the 2 methods used to assay root injury were in perfect agreement might be explained by the large degree of direct freezing injury to leaves in that cultivar.
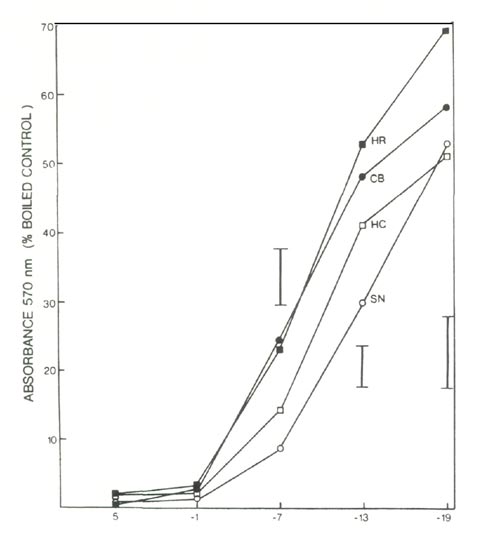
|
|
Fig. 2. Ninhydrin test of freezing injury to roots of 4 azalea
cultivars; see text for cultivar abbreviations. Vertical bars indicate maximum SE at a given temperature, n = 4. |
Desiccation injury
.
Under nursery conditions, desiccation stress commonly occurs on the morning of sunny winter days when container-thawing lags behind rising leaf temperatures (3). In order to separate the effects of low temperature from those of desiccation, it was necessary to determine if the low night temperatures could have any effect on plant water status after thawing.
Freezing and thawing in the dark to temperatures of -10°C and higher did not result in any visible leaf injury for these cultivars. The extent of leaf injury after freezing and thawing in the dark to -14° is shown in Table 1. Stomatal resistance (r s ) in the light at 15°, after a freeze-thaw treatment in the dark, was unaffected by freezing to temperatures which did not result in visible leaf injury (Table 2). The omission of CB from these measurements was due to its small leaf size, which was too small for measurement with the porometer sensor used.
| Table 2. Stomatal resistance and stem water potential in 4 azalea cultivars after freezing and thawing in the dark. | |||||
| Freezing temp. | |||||
| Cultivar | 2°C | -2° | -6° | -10° | -14° |
| Stomatal resistance (sec cm -1 ) | |||||
| Snow | 2.8 | 2.6 | 3.4 | 3.1 | 6.1 |
| Hino Crimson | 3.8 | 2.0 | 3.6 | 3.5 | 5.5 z |
| Hershey's Red | 2.9 | 3.9 | 3.5 | 6.3 | 7.6 z |
| Stem water potential (-bars) | |||||
| Snow | -11.6 | -15.1 | -12.0 | -11.5 | -11.5 |
| Coral Bells | -9.0 | -5.5 | -10.3 | -5.5 | -5.3 |
| Hino Crimson | -13.8 | -13.3 | -7.5 z | -6.0 z | -5.9 z |
| Hershey's Red | -9.1 | -8.6 | -7.4 | -6.9 | -5.0 |
|
z
N = 4, significantly different than controls by Duncan's multiple range test (5% level).
PAR = 75 m -2 sec -1 ; air temperature 15°C, 2 days after freezing. |
|||||
Following freezing and thawing in the dark, there was no significant decrease in ψ in any of the cultivars (Table 2). In contrast, when plants were frozen in the dark but thawed under high light (210 µE m -2 sec -1 ) and high temperature (27°C) conditions, ψ decreased considerably below the unfrozen controls. For example, for plants of HC frozen in the dark to -7° but thawed under the same high light and high temperature conditions, ψ was -11.0 bars in 2 days after freezing, whereas it was -4.0 bars in the unfrozen controls. This difference in ψ persisted long after soil thawing (at 6 days after freezing; -5.3 bars in controls, -11.0 bars in frozen and thawed plants) but evidently resulted from conditions at the beginning of the thawing period when plants in frozen soil were subjected to a high evaporative demand.
Susceptibility of a cultivar to this desiccation stress is affected by the response of transpiration rates to both increasing temperature and soil freezing. In unfrozen soil, transpiration rates in all of these cultivars increased with increasing air temperatures from 16.5 to 24.5°C (Fig. 3). The restriction of water uptake by soil freezing reduced transpiration rates in all cultivars, but at an air temperature of 24.5°, the reduction was substantially greater in the cultivars HC and HR (Fig. 3).
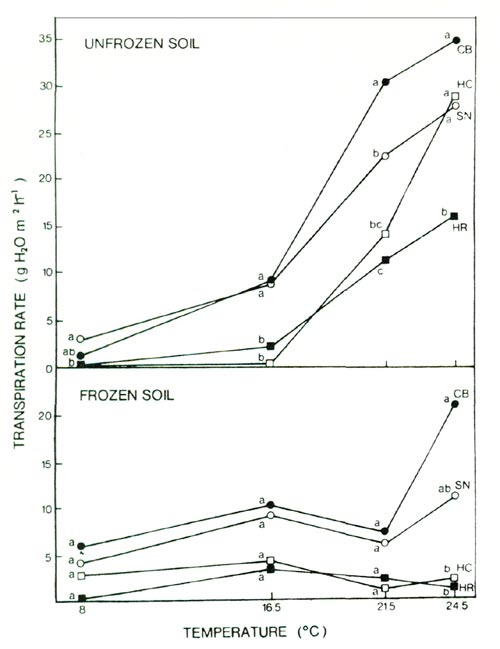
|
|
Fig. 3. Effect of air temperature and soil freezing on
transpiration in 4 azalea cultivars; see text for cultivar abbreviations. PAR, 28 μE m -2 sec -1 ; mean separation within temperatures by Duncan's multiple range test (5% level), n = 4. |
Despite this decrease in transpiration rate, significant water deficits did develop in all of these cultivars under conditions simulating the extreme instances of desiccation stress in the nursery (Table 3). At an air temperature of 27°C, with 210 µE m -2 sec -1 PAR, in unfrozen soil, ψ remained relatively constant over the course of the day in all cultivars, water potentials in SN were about 5 bars lower than the other cultivars. When tops were exposed to the same environment but soil was frozen, ψ was reduced in all cultivars, and decreased throughout the day. There were no apparent differences between cultivars in the extent of wilting after this treatment, but the minimum values of ψ developed can be ranked as lowest in SN, intermediate in CB and HC, and highest in HR.
| Table 3. Desiccation stress in 4 cultivars of azalea. | |||||
| Stem Water potential y (-bars) | |||||
| Treatment |
Time of
day z (HR) |
Snow | Coral Bells | Hino Crimson | Hershey's Red |
| Control | 1000 | -16.0 ± 2.8 | - 9.5 ± 1.4 | - 9.3 ± 1.4 | - 6.5 ± 0.6 |
| 1300 | -12.8 ± 0.9 | - 8.0 ± 0.8 | -10.5 ± 1.6 | - 6.0 ± 1.5 | |
| 1600 | -12.8 ± 1.8 | - 7.0 ± 1.7 | - 6.8 ± 1.5 | - 7.0 ± 1.5 | |
| Soil frozen x | 1000 | -19.9 ± 2.5 | -19.9 ± 2.5 | -11.8 ± 0.9 | -13.5 ± 1.7 |
| 1300 | -22.8 ± 3.8 | -19.3 ± 3.2 | - 19.6 ± 4.3 | - 17.1 ± 1.8 | |
| 1600 | -29.4 ± 4.4 | -24.6 ± 4.6 | -24.5 ± 5.4 | -18.6 ± 2.4 | |
|
z
PAR = 210 μE m
-2
sec
-1
, 0830 to 1630 hr; air temp = 27°C.
y ± SE, n = 4. x Soil temperature = -5°C. |
|||||
Discussion
In order to explore the basis of variation among these cultivars in susceptibility to winter injury, relative susceptibilities to winter injury were compared to the relative degree of injury in response to each of the important stresses separately (Table 4).
| Table 4. Evaluation of winter injury and comparent stress injuries in 4 azalea cultivars. | |
| Type of injury | Relative susceptibility of cultivars z |
| Winter injury in nursery | SN > HC = CB > HR |
| Leaf freezing injury | CB > SN > HC = HR y |
| HR = HC = CB = SN x | |
| Root freezing injury | HR = CB > HC > SN |
| Desiccation injury | SN > HC = CB > HR |
|
z
SN = Snow, CB = Coral Bells, HC = Hino Crimson, HR = Hershey's Red.
y As determined by visual assay. x As determined by triphenyl tetrazolium chloride method. |
|
Direct freezing of roots is the most likely cause of winter injury in containerized plants of many evergreen species (7) due in part to a greater degree of cold hardiness in leaves. This is not the case with these azaleas, however, since the low temperature killing points of roots and leaves are about equal (7). The particular susceptibility of these azaleas to injury during overwintering may be due, in part, to a high susceptibility to freezing injury in the leaves. Freezing injury to leaves, however, does not appear to be a major factor in determining their relative susceptibilities to winter injury. The estimates of relative leaf hardiness to freezing injury in Table 4, based on the results of exposure to -14°C in the visual assay of leaf injury, indicate that HR and HC exhibited the greatest degree of leaf hardiness whereas CB had the least. SN was intermediate in this respect. The TTC failed to corroborate this ranking on a statistical basis although the test data suggests a similar ranking.
The relative susceptibilities of these cultivars to direct freezing injury of roots (Table 4), based on the results of exposure to -14°C in the ninhydrin test and the assay of incremental injury between 2 days and 3 months after freezing, indicate that root injury may not be a problem with these cultivars in the nursery. HR, the cultivar which rarely shows symptoms of winter injury in the nursery, and CB have the greatest susceptibility to root freezing injury; SN, the cultivar most susceptible to winter injury in the nursery, has the greatest hardiness to root freezing injury.
The minimum ψ developed under conditions of frozen soil and high evaporative demand is the single factor which is most closely associated with relative susceptibilities to winter injury. This suggests that desiccation injury is the most important component of winter injury in these azalea cultivars. These estimates of relative susceptibility to winter injury, however, are based on a number of years of observation. Conditions in any particular year can alter the relative importance of desiccation injury and freezing injury to leaves.
Use of the minimum ψ index of susceptibility to desiccation injury leaves a number of unanswered questions about the physiological basis of that variation. Future work on this question needs to be directed towards the relation of ψ with water content in these cultivars. Attention should also be drawn to the evident differences in canopy architecture which may have an important effect on transpiration rates and water contents under desiccation-inducing conditions.
Literature Cited
1. Devenport, D.R. 1980. Factors influencing the winter survival of containerized evergreen azaleas overwintered in unheated polyethylene overwintering structures. MS Thesis, Cornell Univ., Ithaca, N.Y.
2. Fahey, T.J. 1979. The effect of night frost on the transpiration of Pinus contorta spp. latifolia . Ecol. Plant. 14:483-490.
3.Good, G.L. 1977. Principles of storing containerized and balled and burlapped plants under polyethylene structures, p. 30-32. In: Woody Ornamentals Winter Storage Symp., Cooperative Extension Service, Ohio State Univ., Columbus.
4. Havis, J.R. 1965. Desiccation as a factor in winter injury of rhododendron. J. Amer. Soc. Hort. Sci. 86:764-769.
5. Havis, J.R. and R.D. Fitzgerald. 1976. Winter storage of nursery plants Univ. Mass. & County Extension Service, Pub. 125.
6. Steponkus, P.L. and F.O. Lanphear. 1967. Refinement of the triphenyl tetrazolium chloride method of determining cold injury. Plant Physiol. 42:1423-1426.
7. Studer, E.J., P.L. Steponkus, G.L. Good, and S.C. Wiest. 1978. Root hardiness of container-grown ornamentals. HortScience 13:172-174.
8. Wiest, S.C., G.L. Good, and P.L. Steponkus. 1976. Evaluation of root viability following freezing by the release of ninhydrin-reactive compounds. HortScience 11:197-199.
