JARS v40n1 - Rhododendron: An Intimate Glimpse Into the Flower
Rhododendron: An Intimate Glimpse Into the Flower
Barbara F. Palser, Visiting Research Fellow
Plant Cell Biology Research Centre
School of Botany University of Melbourne
Parkville, Victoria, Australia
Reprinted with permission from The Rhododendron, Australian Rhododendron Society, 1985.
"Every once in a while a gem of knowledge is given to us. We were fortunate to receive such a gem on March 15th when Professor Barbara Palser, late of Rutgers University, U.S.A. presented to a gathering of Australian Rhododendron Society members at the Camberwell (Victoria) Civic Centre, the third in the series of Baron F. von Mueller Memorial Lectures."
Lionel Marshall, President, The Australian Rhododendron Society
For the Australian Rhododendron Society to name their special lecture for Baron Ferdinand von Mueller seems eminently appropriate. Von Mueller was an Australian known world-wide among plant taxonomists; he was closely associated with Melbourne, having been Director of the Botanic Garden as well as Government Botanist for a number of years, and he provided the original description and name for many species, several of which were rhododendrons, including the one Australian species; the notation F. Muell. after any scientific name identifies him as the authority for that particular species. It is a distinct honor and privilege for me, as a visiting American, to be asked to present the third of the lectures commemorating this distinguished botanist.
Rhododendron
, of the family Ericaceae, is a very large genus, numbering approximately 800 species, a fact well-known to those interested in the group. In its natural habitats it occurs primarily in the northern hemisphere, particularly southern and eastern Asia, reaching the tropics only in Malesia, with one species,
R. lochiae
, spilling southward into northeastern Australia. In spite of its absence in the wild in the southern hemisphere, many species are widely cultivated in Australia and New Zealand, as well as in many areas north of the equator. As is not surprising in so large a genus, it is very variable in both vegetative and floral characteristics. The objective of the present paper is to review some of this variation, very sketchily for aspects of the vegetative plant and inflorescence (flower cluster), which are the features better known to individuals growing
Rhododendron
. The coverage will be more thorough with respect to the flower and its organs, and the final section will be devoted to consideration of the role the flower plays in the life of the plant.
Rhododendron
plants are best known as terrestrial shrubs, that is, they are rooted in the ground. In some high rainfall areas, particularly in the tropics, however, many species grow as epiphytes where they occur on the trunks or branches of forest trees anywhere from 0.05 to 20 meters above the ground. In spite of the preponderance of moderate-sized plants, from 0.3 to 4 meters in height, there are species, particularly in alpine and arctic areas, which grow essentially as creeping mats not over 10-12 centimeters tall (e.g.,
R. lapponicum
RR
1
R. anagalliflorum
RV,
R. redowskianum
Th. At the other extreme are a number of species (for example,
R. arboreum
HP,
R. macabeanum
HP,
R. falconeri
HP) which form small trees. The majority of species are evergreen, but there are a number of the azalea group that are deciduous (e.g.,
R. reticulatum
TB,
R. calendulaceum
PP,
R. canadense
PR). Many of these produce their flowers before, or concurrently with, the development of new leaves. These features of growth form, place of rooting, and retention, or not, of leaves the year round are used in recognizing species and in the classification of
Rhododendron
that was the subject of the second Baron von Mueller lecture, given by John Rouse (see
The Rhododendron
, Australian Rhododendron Society, Vol. 20 No. 11981), although they do not receive the major emphasis.
1 Each species given as an example, or in an illustration, is identified as to the subgenus and section in which it is classed by letters given after the name (see References for taxonomic sources): AA — subgenus Azaleastrum , section Azalestrum ; AC — Azaleastrum , Choniastrum ; C — Candidastrum ; HP — Hymenanihes , Ponticum ; PP — Pentanthera , Pentanthera ; PR — Pentanthera , Rhodora ; PS — Pentanthera , Sciadorhodion ; RP — Rhododendron , Pogonanthum ; RR — Rhododendron , Rhododendron ; RV — Rhododendron , Vireya ; TB — Tsutsusi , Brachycalyx ; Th — Therorhodion ; TT — Tsutsusi , Tsutsusi .
Considerably more attention in classification is given to the leaf, particularly to the hairs of its under surface, and to the inflorescence and the flowers that make up that inflorescence. A few species, such as R. virgatum RR, R. albiflorum C, and R. ellipticum AC, produce their flowers singly in the axils of leaves below the terminal bud and the latter, when it grows, forms new stem and leaves, In contrast, the terminal bud is floral in most species, developing into the frequently spectacular, few to many-flowered truss which is the hallmark of the genus. In these, new vegetative growth develops from lateral buds below the flowers.
The emphasis in this paper will be on variation in the individual flower and the various organs that make it up rather than on the plant or inflorescence. Many people are familiar with some, if not all of the four floral organs: sepals on the outside, followed, as one goes inward, by petals, stamens and a pistil in the center (Fig. 1). As a whole, the individual flower differs considerably in size from species to species, both in length and in width across the spread petals. Small flowers, 1 centimeter or less in length and 0.5-1 centimeter in width, are found in such species as R. trichostomum RP, R. anagalliflorum RV and R. micranthum RR. There are many of intermediate sizes but at the opposite extreme are species with very large flowers, for example, R. nuttallii RR, R. leucogigas RV or R. griffithianum HP; the latter may be as much as 15 centimeters across the face. A variable much more important than flower size to the plant in the wild is flower shape. Although all organs may be affected by differences in shape, the variation is seen primarily in the corolla (all the petals considered collectively). Not only are the petals the largest organs of the flower but they are also colored so that the corolla constitutes the most conspicuous part. All petals of a Rhododendron flower are joined to one another laterally for varying distances at the base, forming a more or less obvious corolla tube. Flowers of many species are slightly asymmetric and many are funnel-form in shape (Figs. 1, 6, 9) (e.g., R. reticulatum TB, R. ponticum HP, R. luteum PP). There are species, however, such as R. aberconwayi HP or R. souliei HP in which the flowers are much shallower than this so are saucer-shaped (Fig. 4) and still others, including R. nuttallii RR and R. boothii HP where they have a moderately long but very wide tube, are broadly bell-shaped (Fig. 7). Particularly among the Vireyas, the corolla tube tends to be narrow, although its length varies greatly from one species to another. In some where it is rather broad and short (e.g., R. aequabile ) the appearance is rather like that in species in other sections of the genus, but at the other extreme where the tube is very long and narrow (e.g., R. majus , R. herzogii , R. multinervium ) the trumpet-shaped flowers (fig 5) are very distinctive in appearance; the majority of Vireyas fall between these two extremes in tube length. The tube may be straight or curved up or down. In most, the free petal lobes are rather long and broad and bend fairly sharply away from the outer end of the tube so that the flower has an open-faced look (e.g., R. zoelleri , R. majus ) (Fig. 5) but in a few (as R. bagobonum , R. quadrasianum ) the lobes are short and may remain fairly straight so that the flower as a whole is tubular (Fig. 8). Flowers in sections other than Vireya may also have a rather long narrow tube ( R. stamineum AC) or be tubular ( R. keysii RR).
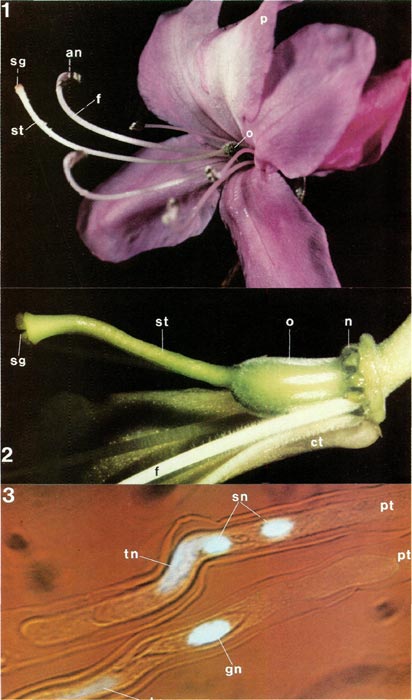
|
|
Figs. 1-3. Fig 1, flower of
Rhododendron reticulatum
TB showing petals, stamens
(anther and filament) and pistil (style, stigma and ovary); sepals not visible. Fig. 2, dissected flower of R. intranervatum RV with whole pistil and nectary, part of corolla tube, and some filaments. Fig. 3, cultured pollen tubes of R. macgregoriae RV showing nuclei, tip of tubes at left; lower tube has tube and generative nuclei, upper one tube and sperm nuclei, an, anther; ct, corolla tube; f, filament; gn, generative nucleus; n, nectary; o, ovary; p, petal; pt, pollen tube; sg, stigma; sn, sperm nucleus; st, style; tn, tube nucleus. Photos by Barbara F. Palser |
The individual floral organs differ greatly from one another in appearance and in function. The sepals are the most leaf-like because they are usually green as well as flattened. In some species of Rhododendron they are moderate in size (Figs. 17-19), but in many they are considerably reduced (Figs. 6, 7, 9) and in others essentially lacking (Figs. 2, 5, 8). Since sepals are organs that are primarily protective, particularly in the bud, their reduction in Rhododendron may be related to the fact that he flower buds in most members of this genus develop within an inflorescence bud where they are protected by large and rather tough bud scales.
The petals have already been touched on in connection with the shape of the flower; but other aspects should be considered. Because of its size, color and, often, odor, the corolla is what is frequently thought of as "the flower". Size has already been referred to, but color only indirectly. The wide variety of colors in the genus contributes strongly to its popularity in cultivation. In a number of species the corolla is pure white (e.g., R. herzogii RV, R. lindleyi RR, R. quinquefolium PS) with variation from this through cream, yellow (e.g., R. luteum PP, R. sessilifolium RV), apricots and oranges (e.g., R. zoelleri RV, R. javanicum RV, R. dichroanthum HP) into the red range: pinks of various shades (as R. lochiae RV, R. roseum PP, R. serpyllifolium TT), reds ( R. buxifolium RV, R. arboreum HP) and deep reds ( R. wrightianum var. wrightianum RV). In a considerable number of species some blue is added to the red range so that the corolla is lavender, magenta (e.g., R. ponticum HP, R. impeditum RR, R. catawbiense HP) (Fig. 1) or even purple (e.g., R. polylepis RR) and R. augustinii RR is often called blue, although it is not a clear blue but has a slight lavender cast. In many species the color is the same throughout the corolla but in others it may vary from one area to another either in actual color or shade of the basic color. One particularly obvious characteristic found in a number of species in sections other than Vireya is the presence of splotches of a different color or of a darker shade on the upper part of the corolla near the mouth of the tube (e.g., R. ponticum HP, R. ellipticum AC, R. augustinii RR), (Figs. 4, 9). The shape, color and odor of a corolla are important to the plant for completion of its life cycle and the variations are associated with specific mechanisms involved.
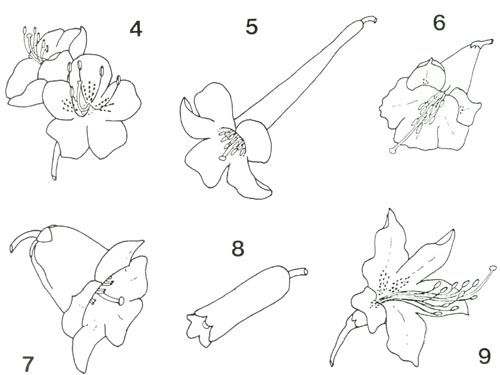
|
|
Figs. 4-9. Diagrams of different flower shapes and stamen arrangements in
Rhododendron
.
Fig. 4, saucer-shaped with style and stamens in upper part of flower ( R. ovatum AA). Fig. 5, trumpet-shaped ( R. majus RV). Fig. 6, funnel-form with central style and evenly distributed stamens( R. rubiginosum RR). Fig. 7, bell-shaped ( R. nuttallii RR). Fig. 8, tubular ( R. bagobonum RV). Fig. 9, funnel-form with style and stamens in lower part of flower ( R. augustinii RR). |
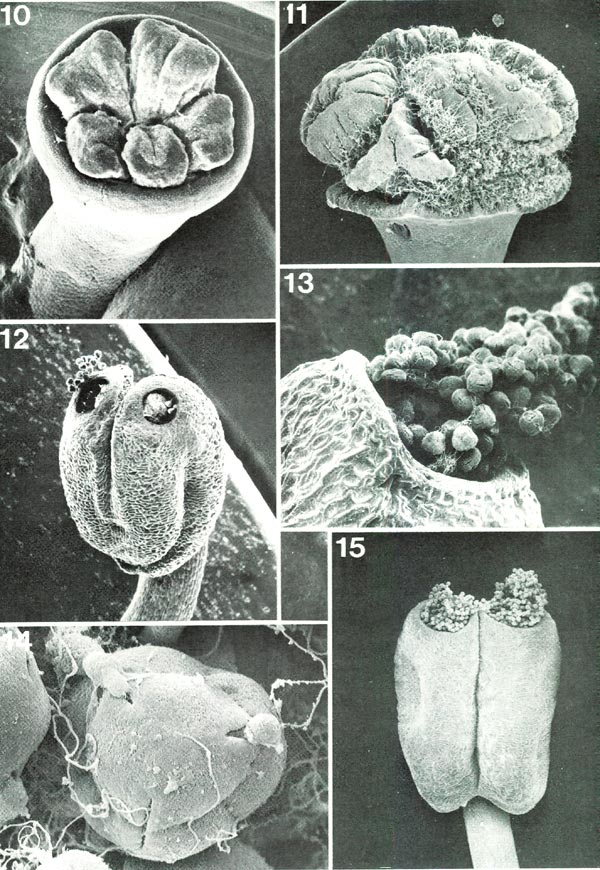
The stamens, though much less conspicuous than the corolla, can normally be seen for much, or at least part, of their length with relatively little difficulty (Fig. 1). They are much narrower than the petals and may, or may not, be pigmented. Although not particularly obvious, the stamen plays a vital role in the life of the plant, a role to be discussed later. Each is composed of two easily distinguishable parts: a narrow filament attached at the base of the flower just internal to the corolla (Figs 2, 19) and, at its outer end, an enlarged anther (Figs. 12, 15). Anthers, although they vary considerably in size and shape among different species, all have certain features in common (Figs. 12, 15). Each is attached on its outer side to the filament and is composed of two rather distinct halves, both of which at maturity have an open pore at the top. Through these pores many small bodies, the pollen grains, are shed (Figs. 12, 13, 15). Stamens range in number from five per flower in several species in section Pogonanthum and in some of the azalea groups (Fig. 1) to ten in many species and occasionally more (as many as 25), particularly in subgenus Hymenanthes . At the base they are attached in regular order around the center of the flower and in some species, as R. macgregoriae RV and R. anagalliflorum RV, they remain so distributed throughout their length (Fig. 6). In other species, however, some of the filaments bend so that the anthers appear clustered in either the upper part (e.g., R. lepthanthum RV, R. ovatum AA) (Fig. 4) or lower part (e.g., R. gracilentum RV, R. konori RV, R. schlippenbachii PS) (Fig. 9) of the flower.
|
|
Figs. 10-15. Scanning electron micrographs of
Rhododendron
stigmas, anthers and pollen.
Fig. 10, R. schlippenbachii PS, un-pollinated stigma seen from above, x34. Fig. 11, R. williamsii RV, naturally pollinated stigma with pollen tubes, seen from side, x19. Fig. 12, R. lapponicum R RR, anther with pollen showing in pores, x56. Fig. 13, R. kawakamii RV, one anther pore with protruding pollen held together by viscin threads, x156. Fig. 14, R. fortunei HP, single tetrad pollen grain with viscin threads and pollen tubes just starting to grow from two upper apertures, x1220. Fig. 15, R. maximum HP, anther with pollen masses protruding from pores, x28. Barbara F. Palser photos |
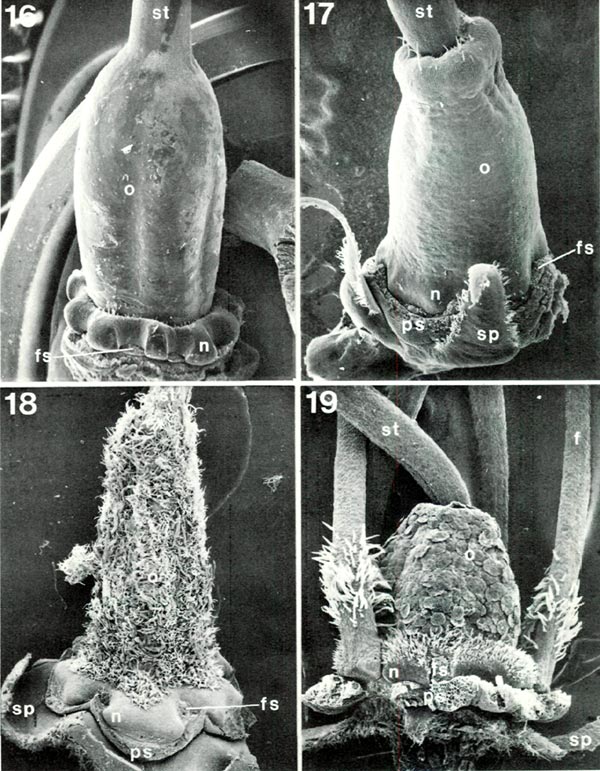
In the very center is the pistil (Figs. 1, 2), not easily seen for much of its length but highly critical nonetheless. It characteristically has three distinct parts (Fig. 2): a basal enlarged portion, the ovary, topped by a narrower, variously elongated style which terminates in a more or less expanded stigma. The ovary is commonly green and the style and stigma may be also, but one or both of the latter may be pigmented. The style may be straight or may be curved in agreement (Figs. 4, 9) with the stamen filaments in the same flower (rarely in the opposite direction as in R. trichocladum RR or R. moupinense RR). Closely associated with the lower end of the ovary is a nectary which bulges to varying degrees between the bases of the filaments (Figs. 2, 19). The size and shape of ovary and nectary can be very different from species to species. Most commonly the ovary is moderately long (Figs. 16, 17) but may be quite short (Fig. 19) or highly elongated. In the Vireyas, and a very few species of other groups, the ovary tapers into the style (Figs. 2, 16). Along with this the nectary is frequently, though not always, a prominent and often deeply lobed ring (as in R. javanicum , R. intranervatum , R. aequabile ) (Figs. 2, 16). In contrast, in almost all species of sections other than Vireya, there is a depression in the top of the ovary and the style emerges from the center of the depression (Figs. 17-19). The conspicuousness of the nectary in these other sections varies, often being rather large in Hymenanthes (Fig. 18), moderately so in Rhododendron (Fig. 19), and only slightly protruding in the azalea groups (Fig. 17), but it is consistently present. The shape of the top of the ovary is easily recognized only in some species - those that either lack, or have very few, hairs, or where hairs that are present are short (Figs. 16, 17, 19). Most species are characterized by a variably dense covering (indumentum) of one or more types of hairs (Figs. 1, 2, 18, 19).
|
|
Figs. 16-19. Scanning electron micrographs of
Rhododendron
ovaries with nectary and base of
style. f. filament; fs. filament scar; n, nectary; o. ovary; ps, petal scar; sp, sepal; st. style. Fig. 16. R. javanicum var. schadenbergii RV. x10; ovary tapers into style and nectary is a distinct, conspicuously lobed ring around base of ovary. Fig. 17. R. quinquefolium PS, x19; style emerges from apical depression in ovary and nectary consists of slight swellings of ovary base. Fig. 18 R. neriiflorum HP, x16; style emerges from depression obscured by ovary indumentum of branched and glandular hairs; paired nectary lobes spread widely from ovary base, Fig. 19. R. lapponicum RR. x32; style emerges from depression; ovary with smooth-margined scales which hide short unicellular hairs; nectary is an obvious bulge of ovary base with unicellular hairs on upper half; some filaments, with unicellular hairs, present. Barbara F. Palser photos |
This introduces the subject of the indumentum which is so heavily emphasized in classification of the genus. The hairs considered for taxonomic purposes are those that occur on the leaf, particularly on its under surface. Hairs are not restricted to vegetative parts of the plant, however, but also occur on the different floral organs with a more or less close correspondence, depending on the organ, to those that are found on the leaves. Hairs of five distinct types (often further subdivided and provided with a multitude of descriptive names) can be recognized. The least variable organs are the stamens and nectary. On the former, hairs commonly occur on the lower half of the filament above the nectary level (Figs. 2, 19) and rarely on the anther. These hairs are unicellular, that is, one-celled extensions from the surface, often rather long. Unicellular hairs also occur on the nectary of almost half the species, primarily on the upper part only (Fig. 19); these hairs vary from extremely short to moderately long. Occasionally a few of the type of hair found on the ovary may spill over onto the very top of the nectary; there they may be the only type present or be mixed with unicellular ones. Hairs of all types may be found on sepals and/or petals, the frequency and type(s) depending on the species.
As already suggested, the ovary commonly has an indumentum, often dense, but because of its usually inconspicuous position in the flower, its hairiness is much less obvious than that of the leaves. The style may also have hairs, though less commonly than the ovary. When present on the style, hairs often, but not always, correspond closely to those on the ovary and are frequently restricted to the lower half. While unicellular hairs do occur on ovaries of quite a few species, they are usually much less conspicuous than hairs of the other four types. Different species of Rhododendron are often spoken of as being lepidote or non- or elepidote. This distinction is based on the presence, in lepidote species, of a particular type of hair usually called a scale. An ovary having the common type of scale appears to be covered with small, essentially circular, almost flat, and frequently overlapping plates (Fig. 19). A sectional, rather than surface, view shows the individual scale to be mushroom- or umbrella-shaped with a narrow stalk extending out from the ovary surface with the plate centered at a right angle on its outer end. Although all scales have the same basic organization, variation occurs in several aspects, the most easily seen being the length of the stalk (very short to quite long), and both margin and thickness of the plate. In many species (e.g., R. micranthum RR, R. hanceanum RR) the margin is essentially smooth (Figs. 22, 25), but in others it is lacerate to varying degrees (e.g., R. zollingeri RV, R. gracilentum RV) (Figs. 21, 24), while in some it is so deeply divided that the scale is star-shaped (stellate) (Fig. 20) as in R. leptanthum RV, R. dielsianum RV, etc. In R. leucaspis RR and some other species the scale cap becomes bloated (Fig. 22) so that the ovary appears to be covered with small pillows. Often scales are the only type of hair present on the ovary (Figs. 20-22), but they may be accompanied by unicellular hairs. The latter may be so short as to be completely concealed by the scales as in R. yunnanense RR and R. lapponicum RR (Fig. 19) or, especially in some of the Vireyas, such as R. vidalii (Fig. 24), they are so long and so abundant as to obscure the scales. Occasionally, hairs and scales are equally conspicuous (e.g., R. scabrifolium RR) (Fig. 25).
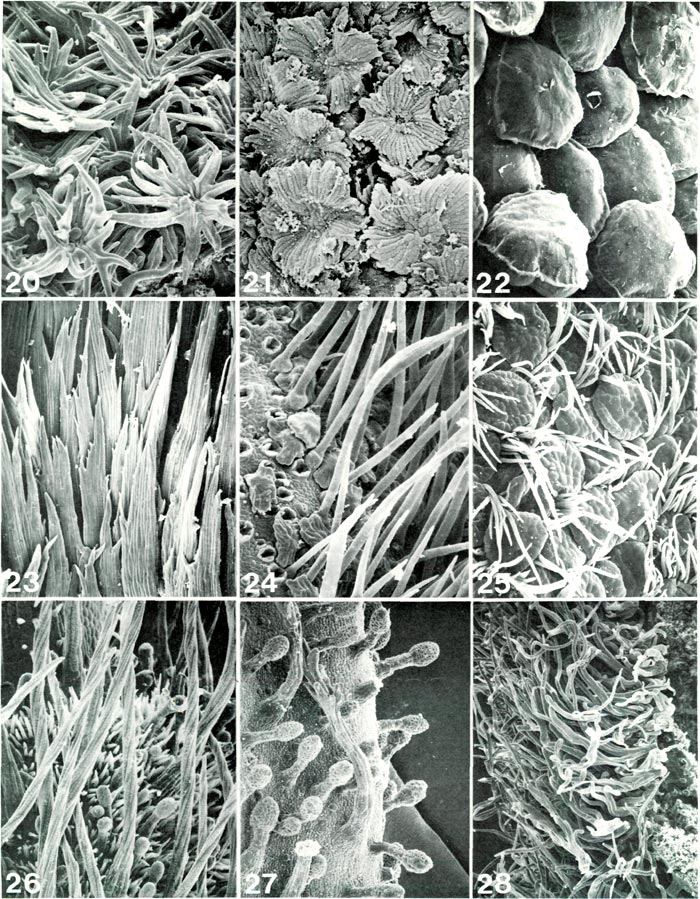
|
|
Figs. 20-28. Scanning electron micrographs of
Rhododendron
ovary hairs. Fig. 20.
R. leptanthum
RV, stellate scales, x190.
Fig. 21. R. zollingeri RV, lacerate scales, x182. Fig. 22. R. leucaspis RV, bloated scales, x190. Fig. 23. R. subsessile TT, flattened, multi-cellular multi-seriate hairs, x104. Fig. 24. R. vidalii RV, small scales with irregular margin obscured by long unicellular hairs, x190; holes at left are bases of hairs which were broken off to expose scales. Fig. 25. R. scabrifolium RR, smooth-margined scales and unicellular hairs, x113. Fig. 26. R. canadense PR, round, twisted, multi-cellular multi-seriate hairs with many unicellular and glandular hairs, x 121. Fig. 27. R. campylocarpum HP, glandular hairs with two moderately small branched hairs, x 121. Fig. 28. R. wiltonii HP, branched hairs with long free branches intertwined, x 86. Barbara F. Palser photos |
Subgenus
Rhododendron
incorporates all of the lepidote species and none of the other subgenera,
Hymenanthes
or any of the azalea groups, has scales. Instead, the other three types of hairs (in addition to unicellular ones) may be found, depending on the species. One of the most common types in these subgenera, occurring on the ovary in many species of both
Hymenanthes
and the azaleas, is the glandular hair. It is called glandular because it secretes a fluid, often sticky. Each of these hairs contains many cells arranged to form a stalk supporting the glandular head (Figs. 26, 27). The stalks vary considerably in length, from very short or essentially missing, through many intermediate lengths, to very long, and the head shows different shapes - round, elliptical, conical - and may be little or clearly broader than the stalk. Particularly among the azaleas glandular hairs may be accompanied by unicellular ones (Fig. 26).
In
Hymenanthes
, and only in this subgenus though not in all its species, a fourth type of hair occurs, a branched one. These have a multi-cellular stalk, frequently very or fairly short, with cells at the outer end long and diverging more or less from one another as branches. When these hairs are moderately small, as in
R. caucasicum
or
R. maximum
, the divergence of the tips is often not great (Fig. 27), but in other species the free terminal cells are very long and intertwine among the end cells of adjacent branched hairs (Fig. 28) to form a dense rough fur on the surface of the ovary as in
R. yakushimanum
,
R. wiltonii
and
R. macabeanum
. In still other species, as
R. rufum
, the stalk of the branched hair is very short and the branches somewhat bloated and widely divergent giving a very different appearance. Branched and glandular hairs occur together frequently (
R. caucasicum
,
R. neriiflorum
- Fig. 18,
R. campylocarpum
- Fig. 27, etc.) and occasionally there may also be unicellular hairs with them, as in
R. maximum
.
The fifth general type of hair is almost completely restricted to the azalea groups as far as its occurrence on the ovary is concerned, though it may be found on leaves or sepals in other groups. This hair is usually long and is made up of many cells in both diameter and length (therefore multi-seriate multi-cellular); in appearance it is much like the stalk of a long glandular hair except that it usually tapers toward the tip and completely lacks the gland. In section Tsutsusi , which includes many Japanese azaleas, these multi-seriate hairs are flattened (e.g., R. serpyllifolium , R. kiusianum ) (Fig. 23) but in sections Brachycalyx and Pentanthera , the latter including many of the American azaleas, they are rather round in cross section and often present a rather twisted appearance (e.g., R. viscosum PP, R. weyrichii TB) (Fig. 26). As will have become apparent, ovaries may have a single type of hair, two types or even three. One species with three, including the round multi-seriate type just described, is R. canadense PR, the other two hair types being glandular and unicellular (Fig 26). Only rarely does the unicellular hair constitute the entire ovary indumentum (e.g. R. intranervatum RV) (Fig. 2) and as indicated at the start of discussion of hairs, only a few species lack hairs completely (e.g. R. javanicum RV, R. quinquefolium PS, R. ponticum HP) (Figs. 16, 17).
The external appearance of the flower with its sepals, corolla, stamens and pistil-nectary, variable and interesting as it may be, gives little or no clue as to the role the flower really plays in the life of the plant. Individuals who are interested in developing new varieties for horticultural purposes know that pollen must be transferred from the anther of one flower to the stigma of a pistil in another flower; if the transfer is successful, seeds will eventually be present in the fruit which develops from the ovary of the pistil to which the pollen was transferred. The questions posed by this procedure are: what happens when pollen is placed on a stigma and what happens between the pollen transfer and the presence of seeds? The answer to these questions can be found only in an understanding of how the plants reproduce.
Flowering plants, including Rhododendron , have sexual reproduction just as animals do. Because plants are non-motile and their body structure is so different from that of animals, the means by which this is accomplished are quite distinct, although the essential part, the fusion of a sperm cell (male) with an egg (female), is the same in both. This is one aspect of a Rhododendron plant about which most people who grow them for their personal enjoyment, or even for commercial purposes, know relatively little. The remainder of this paper includes a general and relatively non-technical description of what happens in a flower that leads eventually to the formation of seeds.
I will start by asserting that stamens are the male organs of flowering plants and pistils the female organs and trust that it will be apparent by the end of the description that this assertion is justified. There are species, of which the date palm is an example, in which one tree has flowers with pistils but no stamens (pistillate or female flowers) and an entirely different tree has flowers with stamens but no pistils (staminate or male flowers). Even the ancient Egyptians recognized, without knowing why, that they had to have both types of tree present to obtain any fruit. Still other species, such as corn, have pistillate flowers on one part of the plant (the ear — with the silks being the style-stigmas of many flowers) and staminate flowers in another place on the same plant (the tassel). As already seen, however, stamens and pistil occur together in each Rhododendron flower, as they do in the majority of flowering plants: thus it appears that the two sexes are at least in close proximity to one another. In plants, however, just as in animals, it is usually best for matings not to occur within the same "family" (i.e., within a single flower or between flowers on the same plant - called "selfing") but rather to occur between flowers on different plants of the same species (called "crossing"). Plants have evolved several mechanisms to insure that, under normal conditions, at least a large proportion of cross-matings rather than self-matings occur. In species with both sexes in a single flower these include differences in time of maturation of stamens and pistils (true in most Rhododendron species), differences in arrangement of sismens and pistils within different flowers of the same species and various chemical mechanisms ("self-incompatibility"). If cross-mating is best and most common, how do stamens of one Rhododendron flower have anything to do with the pistil of a flower on a different plant?
The outward appearance of the stamen has already been considered: a narrow filament with, at its outer end, an enlarged anther (Figs. 1, 12, 15) containing many small rounded bodies called "pollen grains" (Fig. 13). In Rhododendron , as in other members of the Ericaceae, the mature pollen is unusual for flowering plants in general in consisting of four individual grains which adhere closely to one another (thus, a compound or tetrad grain) (Figs. 0, 13, 14). There are lens-shaped openings (apertures) in the pollen wall, any one cell of the tetrad sharing an aperture with each of the three adjacent cells. In addition, Rhododendron , and a few of its closest relatives in the Ericaceae, has, scattered rather abundantly among the pollen grains, long narrow "viscin" threads attached at one end to a pollen wall (Figs. 0, 14). These serve to hold most of the pollen in one anther sac together in a loose irregular mass (Fig. 13). Such masses can be seen dribbling out of the pores of open anthers (Figs. 12, 15) and greatly facilitate the transfer of considerable quantities of pollen at a single touch. So, although the anthers are stationary, they do contain pollen grains which are small and, while not mobile themselves, might easily be moved.
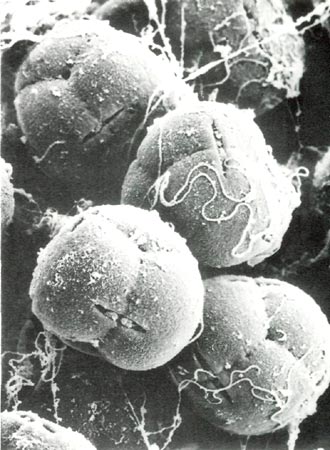
|
|
Fig. 0. Scanning electron micrograph of pollen grains of
Rhododendron fortunei
HP showing tetrad
arrangement, viscin threads and lens-shaped apertures, x1160; bulges in some apertures are incipient pollen tubes. A scanning electron microscope uses a beam of electrons, rather than regular light, to view and photographically record the surface features of a specimen which has been coated with an extremely thin layer of gold. Photo by Barbara F. Palser |
If there is movement of pollen under natural conditions, how is it accomplished? Wind would appear to be an obvious answer and it is the answer for some plants, including the grasses, but it is not the answer for Rhododendron . If a grass flower is compared to that in Rhododendron , it will be seen to be very different: it is small, though several flowers may be clustered together; it completely lacks the large brightly colored corolla, and the most conspicuous flower parts are the stamens in which large, longitudinally opening anthers containing very large numbers of single pollen grains are borne at the ends of very long, extremely thin filaments. Thus the anthers project well out into the air and are very easily shaken by even slight wind currents. Stigmas are dry and feathery. In contrast, Rhododendron with its large flowers, conspicuous corolla, sturdy filaments, tetrad pollen grains, terminal pores on the anthers and rather solid sticky stigma, relies on animals to move its pollen. The particular animal varies; it may be a bee, a butterfly or moth, a bird, or occasionally in home gardens, man. Why should any animal, such as a bee, visit a flower? I think it is safe to assume that it is not "out of the goodness of its heart" nor a desire to perform a necessary chore for the plant. It goes because the flower supplies it with something that it needs. Rhododendron offers rewards of two types, either one or both of which may serve to bring a particular type of animal to its flower. One is the pollen itself. Pollen is rich in proteins and frequently lipids (fatty substances) or starch as well, and a bee actively collects pollen to provide food for the developing young in the hive. In addition, as already pointed out, a nectary is closely associated with the base of the ovary. At about the time the flower opens the nectary secretes a fluid, nectar, which usually contains a considerable amount of sugars: concentrations of from 10-70% have been measured in various plants and are often in the 20-30% range, so nectar provides an excellent food source. It is pleasant to suck nectar from the corolla tube of many flowers, but I do not recommend it with Rhododendron . The nectar of several species has been shown to be poisonous to humans, though it is not toxic to the birds and the bees.
In visiting a flower for food a bee brushes against one or more open anthers and some pollen sticks to the hairs covering its body. As it moves away, more pollen is pulled out of the anther because of the viscin threads among the grains. In the same or often in a different flower the bee brushes against the stigma and some of the pollen on its hairs sticks to that stigma (Fig. 11). Thus, pollen has been moved from the anther of one flower to the stigma of the same or of another flower; this is called "pollination". The color, shape, and odor of flowers are all closely related to the type of animal a particular plant species depends on to effect its pollination. A bee has to land to feed, whereas a hummingbird and many moths hover, so a bee flower is wider open to provide a landing place and a moth, or humming bird, flower is horizontally oriented. Other birds, such as honey eaters, perch when feeding and flowers they visit usually curve downward. Honey bees cannot see red so a red-flowered species is not pollinated by these insects but may be visited by birds. Since most types of bees have short tongues, in flowers depending on bees for pollination the corolla tube, which holds the nectar after it has been secreted, must be broad and/or shallow enough to allow the bee to crawl to the nectar. In contrast, birds and moths frequently have very long tongues and flowers they pollinate are often long-tubed. Color and odor provide recognition signals so that a bee, for example, can move from flowers on one plant to those on another plant of the same kind. The splotches of color on the corolla serve to direct the pollinator to the corolla tube where nectar accumulates and are often called "nectar" or "honey guides".
Before the pollen story can be completed, it is necessary to look in more detail at the individual parts of the pistil: the ovary, style and stigma (Fig. 2). The latter is of immediate interest, of course, because this is where the pollen has been deposited. In Rhododendron the stigma frequently has a groove all around the periphery and always has five or more grooves radiating from the center out to the peripheral one so that the stigma is obviously lobed (Figs. 10, 11). At the time the stigma is receptive, that is, when pollen will adhere to it, it is wet with a sticky fluid and actually glistens. Anyone who wishes to pollinate a flower should check that the stigma is receptive before doing so, or the cross is likely to fail from the outset. Large numbers of pollen grains are often caught in this exudate. Here, within 24 hours, the pollen germinates; each of the four cells of the tetrad grain can produce a tube which emerges through one of the lens-shaped apertures in the wall (Fig. 29), though all four cells do not necessarily do so. Each pollen tube elongates at the tip and usually, probably because of some chemical attraction, heads rather directly for the nearest groove in the stigma and disappears from sight (Fig. 30).
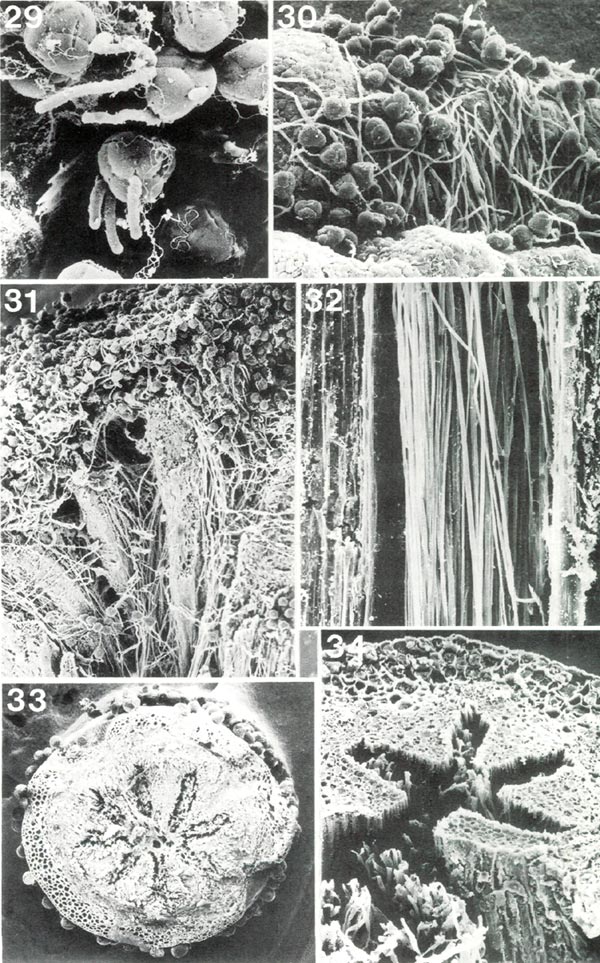
|
|
Figs. 29-34, Scanning electron micrographs of
Rhododendron
pollen tubes on stigma and in style. Figs. 29, 31, 33,
R. fortunei HP; figs. 30, 32, 34, R. ponticum HP. Fig. 29, early germination of pollen grains on stigma, x 350. Fig. 30, germinated pollen on stigma, x 145; pollen tubes have grown down into a stigmatic groove. Fig. 31, stigma and upper style cut lengthwise showing pollen tubes from grains on stigma surface (at top) entering and traversing four arms of stylar canal, x49. Fig.32, longisection of portion of style with pollen tubes in stylar canal, x 280. Fig. 33, cross section of style with stellate stylar canal in center, x 39; "spots" in canal are sections of pollen tubes and "balls" on outer surface are heads of glandular hairs. Fig. 34, combined cross and longisection of style viewed obliquely, x 200; four arms of stylar canal are intact, each with a few pollen tubes; fifth arm is cut; many pollen tubes, pulled out of stylar canal, show in foreground. Barbara F. Palser photos |
To find the fate of the pollen tubes, it is necessary to see what occurs inside both the style and ovary. A cross section of the style shows that, while it is made up of many cells, it is not solid. There is a narrow star-shaped cavity in the center which is called the "stylar canal" (Figs. 33, 34). Upward this canal is continuous with the grooves in the stigma and downward it extends to open areas within the ovary. So far, the ovary description has been restricted to the external appearance without any indication of its internal structure. If a cut is made across the middle at right angles to the long axis, five openings or locules around a central solid core are seen (Fig. 35) (rarely less than five, often more in species of Hymenanthes) . Projecting into each of these locules from the central core is a mass of tissue called the "placenta". This is narrower toward the center and expands outward with many small oval bodies, or ovules, attached around the outer broader part. As will be seen, the ovules are forerunners of the seeds. Each placenta is at least partially separated into two halves by a narrow cleft extending inward from the outer surface. If, instead of cutting an ovary at right angles, the outer wall of a locule is removed, what is usually seen are ends of many ovules because they are borne over the entire placental surface (Fig. 35), but in many species of Hymenanthes ovules occur only on the sides of the placenta and in such species the placental cleft can be seen end-on; it extends from the very top of the locule where the ovary joins the style to or almost to, the base of the placenta (Fig. 36). it is this cleft which is directly continuous with the stylar canal and, thus, also with the grooves in the stigma. There is, therefore, a continuous narrow passage from the surface of the stigma to the locules of the ovary.
Each ovule is a multi-cellular structure; it is longer than broad and attached to the placenta at one side of a smaller end (Figs. 35, 36, 38). The pattern of surface cells suggests that the ovule, from its place of attachment, is bent back on itself with the far end coming back close, but not attaching, to the placenta (Fig. 38). That this is actually the case is seen when ovule development is followed from initiation to maturity. When inversion is complete a very narrow passage, called the "micropyle", is left extending into the ovule from the surface close to the placenta. At the time of pollination the ovule in Vireyas has a somewhat different appearance; its basic structure is the same, but it has already elongated greatly at both ends to form tails (Fig. 37). These make no difference in the function of the ovule.
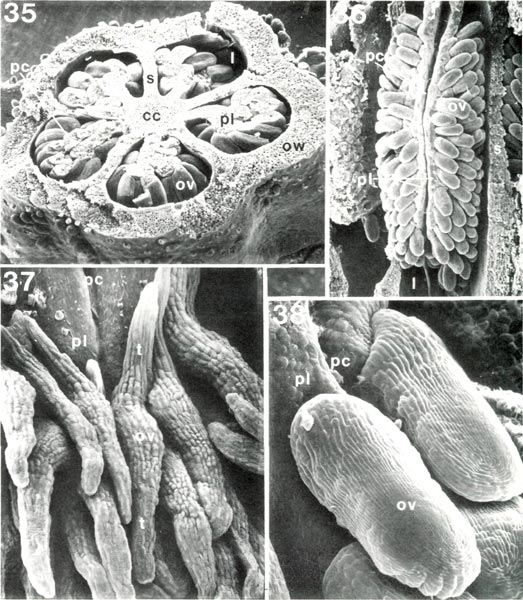
|
|
Figs. 35-38. Scanning electron micrograph of dissected ovaries and ovules of
Rhododendron
, cc, central core;
l, locule; ov, ovule; ow, ovary wall; pc, placental cleft; pl, placenta; s, septum between locules; t, ovule tail. Fig. 35, R. schlippenbachii PS, ovary cut transversely, x24; each of five locules has a cleft placenta with ovules attached all around enlarged outer end. Fig. 36, R. caucasicum HP, ovary locule from which outer wall has been removed, x24, placental cleft extends from top to bottom; ovules borne only on sides of placenta. Fig. 37, R. javanicum var. schadenbergii RV, upper end of placenta with tailed ovules, x134. Fig. 38, R. quinquefolium PS, ovules at upper end of placenta, x170. Barbara F. Palser photos |
|
|
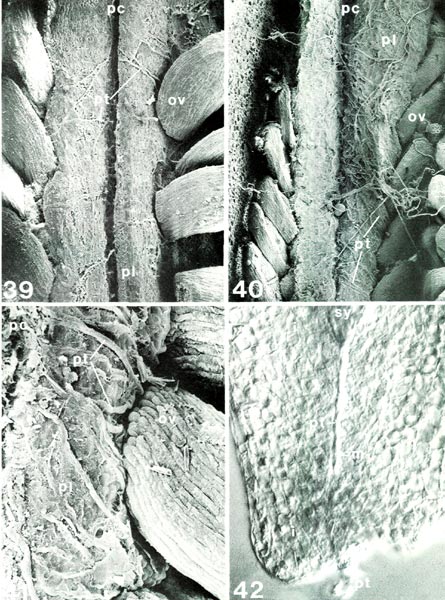
Figs. 39-42.
Rhododendron
pollen tubes in ovary locule and ovule. Figs. 39-41, scanning electron micrographs
of R. fortunei HP; Fig. 42, R. nuttallii RR, i, integument; m, micropyle; ov, ovule; pc, placental cleft; pl, placenta; pt, pollen tube; sy, synergid tip. Fig. 39, longitudinal view of placenta with ovules 7 days after pollination, x132; first pollen tubes have emerged from placental cleft and crossed outer surface of placenta onto sides under ovules. Fig. 40, as fig. 39 except 10 days after pollination, x94; many pollen tubes now present. Fig. 41, half placenta with base of ovule 10 days after pollination, x290; path of individual pollen tubes more easily seen. Fig. 42, micropylar end of cleared whole ovule, placenta would be at bottom, x505; pollen tube extending through micropyle 15 days after pollination; compare with ovule section in figs. 43, 44; micropyle here in R. nuttallii is longer than there in R. yunnanense . Barbara F. Palser photos |
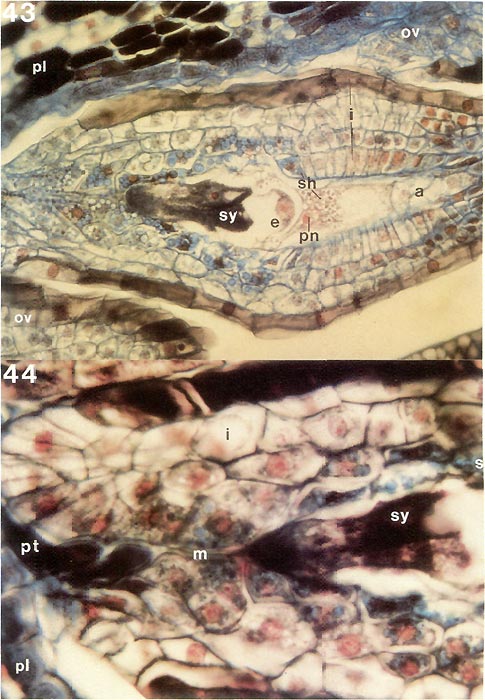
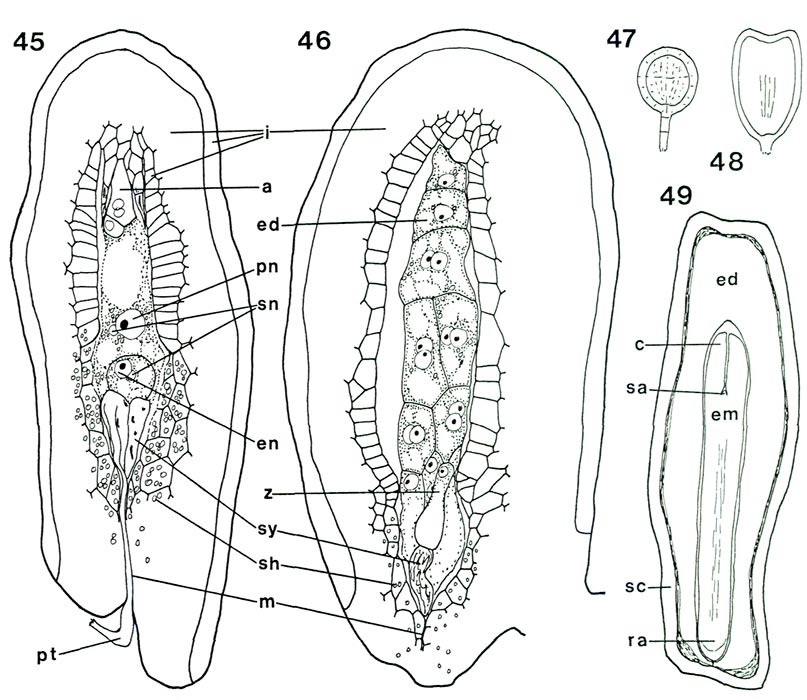
Now that the internal structure of the style and ovary is known, it is possible to follow what happens to the pollen tubes formed by the pollen grains on the surface of the stigma. When they enter a stigmatic groove and disappear from view, they actually enter the stylar canal in which there is an exudate similar to that on the stigma (Figs. 31, 33, 34). They continue to elongate at the tip following a rather straight pathway down through the canal (Fig. 32) and, in R. fortunei , by 7 days after pollination (Fig. 39) have reached the upper half of the ovary where they occur in the placental cleft. There they turn rather abruptly outward and emerge on the outer surface of the placenta, cross over it and pursue a course on the lateral surface among the ovules. With increasing time after pollination, many more pollen tubes reach the ovary (Figs. 40, 41). One pollen tube, or occasionally more, enters an ovule via the micropyle (Figs. 42, 44, 45). This means that there is now a pollen tube, which grew from a pollen grain carried to the stigma from an anther in another flower, inside an ovule within the ovary of the pistil on which the pollen was deposited. A further step must be taken. As already pointed out, the essence of reproduction is the fusion of a sperm with an egg. So far, neither of these important cells has been seen. This means returning, once again, to the pollen. When a pollen grain is stained specifically to show the nuclei inside, two nuclei become visible in each of the four cells of the tetrad. One is small and stains heavily (called the "generative" nucleus); the other is larger and more diffuse in its staining (the "tube" nucleus). When the pollen germinates, the two nuclei migrate into the pollen tube with the tube nucleus leading; there they remain a short way back of the tip of the pollen tube as it grows through the stylar canal (Fig. 3). Somewhere within the style, usually 2-4 days after pollination, the generative nucleus divides to form two (Fig. 3). Each of these new nuclei belongs to a sperm cell and continues to move down the tube as the latter grows. Thus, the pollen tube which enters an ovule contains two sperms. This suggests that there probably is an egg somewhere within the ovule. To see whether this is indeed the case, a thin lengthwise section is cut from the center of an ovule to determine its internal structure. When the section is stained and observed under a microscope, the ovule is seen to have several layers of small cells around the periphery surrounding an elongated, different-looking structure in the center (Fig. 43). The peripheral cells, the integument, are protective and because of the presence of starch in some of the cells, also supply a certain amount of food. The central structure, or embryo sac, is the critical part. It is made up of seven cells: three small ones farthest away from the placenta, called "antipodal cells", a large central cell with two polar nuclei which commonly, in Rhododendron, fuse to form one large secondary nucleus, and three moderately large cells closest to the placenta. One of these latter three, the one less heavily stained and farther from the placenta, is the egg; the other two are synergids. The integument is traversed by the narrow micropylar channel from close to the placenta to the start of the embryo sac (Figs. 44, 45). The pollen tube which goes into an ovule enters the micropyle from the placenta (Fig. 44), grows through it (Fig. 42) into one of the synergids where it extends almost to the inner end of that synergid (Fig. 45). There it breaks open releasing its contents, including the two sperms, in close proximity to the egg. One of the sperms fuses with the egg (Fig. 45), thus initiating a new plant. The fusion of egg and sperm is called "fertilization". The second sperm is not wasted; it moves to, and fuses with, the two polar nuclei (or the one secondary nucleus formed by their fusion) in the central cell (Fig. 45). This is a second fertilization, so Rhododendron , just as other flowering plants, has a double fertilization. The fertilized egg (zygote) remains relatively inactive for awhile, but the fertilized central cell (primary endosperm cell) starts dividing soon after nuclear fusion (Fig. 46). It forms a new tissue called "endosperm", which is important in the nutrition of the new plant derived from the fertilized egg. When the zygote starts to grow, it elongates and the tip goes through a very orderly series of divisions to form initially a rather round, then heart-shaped, structure, the young embryo (Figs. 47, 48), and eventually an elongated miniature plant with a root tip and two seed leaves (cotyledons) with a minute shoot apex between them (Fig. 49). This "mature" embryo, surrounded by remnants of the endosperm and the ovule integument (now seed coat), is the seed (Fig. 49). It is still attached to the placenta inside the ovary.
|
|
Figs. 43, 44. Stained thin longisections of ovule of
Rhododendron yunnanense
RR, placenta
surface at left; a, antipodal cells; e, egg cell; i, integument; m, micropyle; ov, ovule; pl, placenta; pn, polar nucleus; pt, pollen tube; sh, starch. Fig. 43, ovule with mature embryo sac just before entrance of pollen tube; x310. Fig. 44, micropylar half of ovule with two pollen tubes just entering opening of micropyle, x570. For pollen tube extending to synergids see cleared ovule in fig. 42. Barbara F. Palser photos |
|
|
Figs. 45-49. Diagrams of fertilization, developing endosperm and embryo, and seed in
Rhododendron
,
a, antipodal cells; c, cotyledon; ed, endosperm; em, embryo; en, egg nucleus; i, integument; m, micropyle; pn, fused polar nuclei; pt, pollen tube; ra, root apex, sa, shoot apex, sc, seed coat; sh, starch; sn, sperm nucleus; sy, synergid; z, zygote. Fig. 45, R. yunnanense RR. longisection of ovule showing double fertilization (see fig. 43 for ovule of same species with mature embryo sac); remains of pollen tube still visible in micropyle and synergid; synergids disintegrating; one sperm nucleus in contact with egg nucleus, the second with the fused polar nuclei. Fig. 46, R. camtschaticum Th, longisection of post-fertilization ovule; one badly disintegrated synergid present; zygote has elongated slightly; endosperm has formed approximately 16 cells. Fig. 47, round (globular) stage of developing embryo; develops from tip of the zygote after the latter is at least double the length seen in fig. 46. Fig. 48, heart-shaped embryo, cotyledons just initiated. Fig. 49, longisection of seed showing seed coat (remains of ovule integument), endosperm and mature embryo. Barbara F. Palser photos |
The ovary, after pollination and fertilization, remains on the plant, although the stamens and corolla fall off. It enlarges considerably as the ovules inside are developing into seeds. By the time the seeds are mature, the enlarged ovary is called a "fruit"; it has become quite hard and is often brown, rather than green. It then starts to split open, starting at the top, along the lines originally separating the ovary locules. When the segments have begun to spread more widely, the seeds break off from the placenta and fall out of the fruit. If the seeds are required for planting, the fruit should be harvested before it has opened very far or the seeds are likely to be lost. The Rhododendron seed is very small. In many species, such as R. ovatum AA, R. anthopogon RP, R. camtschaticum Th, etc., it is unadorned and has approximately the same shape as the original ovule, although it is clearly larger The seeds of most Vireya species, however, are characterized by tails on both ends; these were already well initiated by the time of fertilization (Fig. 37). In many species the developing seeds (after fertilization) form rather ornate, small to large, appendages at one or both ends (as R. burmanicum RR, R. leucaspis RR, R. racemosum RR). Most commonly when appendages are present there is also a narrow to broad peripheral flattened wing, as in many species of Hymenanthes (e.g., R. irroratum , R. fortunei , R. grande ) and some azaleas (as R. canadense PR, R. luteum PP, R. serpyllifolium TT).
Most Rhododendron seeds will germinate as soon as they are released from the fruit. When they fall or are sown on the top of soil under appropriate conditions, the new plant will start to develop within 2-4 weeks. The root of the embryo will emerge from one end of the seed and grow into the soil; the axis above the root elongates upward and the seed coat is pushed off by the expanding cotyledons which spread apart, enlarge, and become green. At this stage the seedling is often so small that it requires a magnifying lens to see it clearly, but under proper conditions it will continue to enlarge; the originally tiny shoot apex starts to grow and produce stem and new leaves. After a few regular leaves have developed, the cotyledons are lost. The new plant continues to enlarge and eventually, in 3-10 years depending on the species, may produce its first flowers.
Thus, a complete cycle has been followed, starting with an adult Rhododendron plant producing flowers with stamens and pistil and ending with a completely new plant of essentially the same type, also producing flowers with similar organs. This, then, is the answer to what happens to pollen when it is placed on an appropriate stigma and how that pollination leads to seed development and the formation of a new plant. The story is complete enough to understand the elements of reproduction in Rhododendron and other flowering plants, but anyone who thinks about the consequences of repeating the process, as described, generation after generation will recognize a snag and realize that something has been omitted. Rhododendron fanciers who pollinate some of their species often transfer pollen from the flower of one species to the stigma in the flower of a different species in the hope of obtaining a new plant (hybrid) that combines the particularly desirable features of both the pollen and seed parents. In general terms, it can be said that the new plant formed by fertilization carries hereditary characteristics of both the male and female parent. In the scientific sophistication of today, it is rather universally known that the determinants for hereditary traits are carried in the nucleus of a cell. This is just as true for plants as for animals. The fusion of a sperm nucleus with an egg nucleus has brought together the two sets of determinants, one from the male and one from the female parent, so that the zygote, and thus the embryo and new plant which develops from it, carries a double set of hereditary traits. When the new plant, in turn, reproduces, crossing with another formed in the same way, it would appear that the third generation offspring would have a quadruple set of determinants and a fourth generation offspring eight times the number, etc. This continued doubling of nuclear components would lead to total confusion and, in fact, does not occur. Each plant does, indeed, carry two sets of chromosomes (nuclear components which carry the hereditary determinants), one derived from the sperm and one from the egg which initiated it. During the development of the plant produced by fertilization, however, a very special type of division occurs in two places and in only those two places. One is the young anther, in just those particular cells which eventually form the pollen grains and the other is the immature ovule, in just that cell which leads to the development of the embryo sac. The significance of this special division, called "meiosis", is that the nuclear components are halved, so that each of the resulting pollen grains or embryo sacs, and the sperms or eggs which they produce, has only half of the hereditary material of the plant producing the anther or ovary; that is, each has a single set of chromosomes. Thus, when sperm and egg fuse to form the next generation the new plant has only two sets of chromosomes and it makes no difference what generation it may be - first or twenty-first. There is, thus, a regular alternation from one generation of Rhododendron plants to the next: adult plant with a double set of chromosomes, pollen and embryo sac with a single set, then embryo and new plant with a new double set, and so on.
A further question may arise: what about the endosperm which is also initiated by fertilization? The endosperm was called a "new" tissue and it is distinct. Since it results from the fusion of a sperm with the two polar nuclei of the embryo sac, it has a triple set of chromosomes: two maternal and one paternal. Because the endosperm does not persist beyond the seed and has no connection with the plant produced from the seed, it has no effect on the heredity of that new plant. Its importance lies in its role as an intermediary or transfer tissue between parent integumentary tissues and embryo in the developing seed, and in some species, including Rhododendron , in the mature seed it has become a tissue storing food to be used by the embryo during seed germination. Endosperm might, thus, be considered a "nurse" tissue, important but not persisting.
Although pollination has occurred, seeds may not develop for a variety of reasons. Any one of (1) failure of pollen tubes to reach the ovary, or if they do reach the embryo sac, (2) lack of fertilization of egg and/or polar nuclei, or (3) non-development of either embryo or endosperm after fertilization has occurred will normally lead to seed abortion. Failure of interspecific crosses in Rhododendron is usually the result of (1) and poor seed set in many selfed Rhododendron species may be caused by (2) or (3).
Variations in shape of flower, color, odor and even of visiting pollinators are easily recognized by careful observation in the wild or in private or public gardens growing a number of different Rhododendrons. Many of the less well-known aspects of variation, such as the types of hairs, shape and position of anther and stigma, ovary shape, nectary appearance, etc., as well as aspects of the life cycle can be seen by merely taking a flower apart; thinnish sections or cross and lengthwise cuts with a penknife will show further structure, such as number of locules and general placental and ovule features, and a hand lens with reasonable magnification will further facilitate seeing some of the details. Cells, such as egg and sperm will not be visible, but many parts of the reproductive story can be seen with relatively little effort. I recommend careful inspection of floral details when next you have the opportunity; I believe you will find that your enjoyment and appreciation of this popular genus will be considerably enhanced.
The author gratefully acknowledges financial support from the Plant Cell Biology Research Centre which made the stay in Melbourne possible. The many facilities made available and unstinting cooperation of people in the Centre and School of Botany are greatly appreciated.
The illustrations used in this article are part of an ongoing research program and the author retains the right to publish them in scientific articles elsewhere.
REFERENCES
CLASSIFICATION
Ericaceae
Old
Drude, O. 1889. Ericaceae. Pp. 15-65
in
Engler, A. and K. Prantl (Eds.) 1897. Die naürlichen Pfanzenfamilien. IV Teil, 1-2 Abt. Wilhelm Engelmann, Leipig.
Most recent
Stevens, P.F. 1971. A classification of the Ericaceae: subfamilies and tribes. Bot. J. Linn. Soc. 64: 1-53.
Rhododendron
Old
Sleumer, H. 1949. Ein System der Gattung
Rhododendron
L. Bot. Jb. 74:511-553. Translated by H. F. Becker and reprinted, pp. 1-18
in
Luteyn,J. L. and M. E O'Brien (Eds.) 1980. Contributions towards a classification of Rhododendron. New York Botanical Garden, Bronx, NY.
Most recent
Chamberlain, D. F. 1982. A revision of
Rhododendron II
. Subgenus
Hymenanthes
. Notes Roy. Bot. Gard. Edinburgh 39:209-486.
Cullen, J. 1980. A revision of
Rhododendron
. I. Subgenus
Rhododendron
sections
Rhododendron & Pogonanthum
. Notes Roy. Bot. Gard. Edinburgh 39: 1-207.
Philipson, M.N. and W. R. Philipson, 1982. A preliminary synopsis of the genus
Rhododendron
. III. Notes Roy. Bot. Gard. Edinburgh 40: 225-227. (for the azaleas)
Sleumer, H. 1966. Ericaceae.
In
Van Steenis, C.G.G.J. (Ed.) Flora Malesiana, Ser. 1, Vol. 6(4):469-668, Noordhoff, Groningen, (for the Vireyas)
RHODODENDRON HAIRS
Cowan, J. M. 1950. The
Rhododendron
leaf, a study of the epidermal structures. Oliver and Boyd, Edinburgh.
Seithe, A. 1960. Die Haarformen der Gattung
Rhododendron
L. und die Möglichkeit ihrer taxonomische Verwertung. Bot. Jb. 79: 297-393
Seithe, A. 1980.
Rhododendron
hairs and taxonomy. Pp. 89-115
in
Luteyn, J. L. and M. E. O'Brien (Eds.) Contributions toward a classification of
Rhododendron
. New York Botanical Garden, Bronx, NY. (primarily leaf)
Hedgegaard, J. 1980. Morphological studies in the genus
Rhododendron
dealing with fruits, seeds and seedlings and their associated hairs. 2 vols. G.E.C. Gads Publishing House. Copenhagen.
FLOWER IN RELATION TO POLLINATION AND/OR REPRODUCTIVE CYCLE
General — at elementary botany level
Raven, P. H., R. F. Evert and H. Curtis. 1981. Biology of plants. 3rd ed. Worth Publishers, New York, Pp. 356-371, 377-379, 382-397.
General — at more advanced level
Faegri, K. and L. van der Pijl. 1979. The principles of pollination ecolgy. 3rd ed. Pergamon Press, Oxford.
Willson, M. F. 1983. Plant reproduction ecology. John Wiley & Sons, New York.
Specifically
Rhododendron
Stevens, P. F. 1976. The altitudinal and geographical distributions of flower types in
Rhododendron
section
Vireya
, especially in the Papuasian species, and their significance. Bot. J. Linn. Soc, 72: 1-33.
