JARS v44n3 - Rhododendron Propagation
Rhododendron Propagation
John L. Harrington
Newport News, Virginia
Research conducted by others indicates that many factors can effect the results obtained when attempting to root rhododendrons from cuttings (Beley, 1985, Bush-Brown 1958). During earlier research on this subject, the effects of several factors were established; however, further investigations were determined to be required concerning the effects of rooting hormones and the applicability of the results to rhododendrons other than R. 'Roseum Elegans' (Harrington 1987, Harrington 1988).
A four-factor factorial design procedure was used to determine the effects that four different rooting hormones had upon the ability to root cuttings from three significantly different rhododendrons. The results from the experiment showed that the average effects of the use of the rooting hormones, indole-3-acetic acid, indole-3-butyric acid, Rootone, and naphthalene acetic acid, on cutting survival was -18.3%, -13.3%, -10.0%, and +11.7%, respectively. The only beneficial two-factor interaction effect of significance was a +16.7% effect between indole-3-acetic acid and naphthalene acetic acid; however, this effect was of little importance because of the large adverse main effect of indole-3-acetic acid. The grand average survival rate of all cuttings was 85.0%; therefore, the +11.7% beneficial main effect of naphthalene acetic acid was of noteworthy significance.
Test results showed that the rhododendrons R. euchaites , [Syn: R. neriiflorum ssp. euchaites ], R. 'Catawbiense Album,' and R. 'Roseum Elegans' had survival rates of 90.0%, 86.3%, and 78.8%, respectively. The highly favorable conditions that had been established for the propagation of R. 'Roseum Elegans' were, therefore, determined to also be favorable for the other varieties tested.

|
|
Cuttings in propagation area after one winter.
Photo by John L. Harrington |
Introduction
Good sources of information concerning rhododendrons and the ways that they can be propagated are included in the Bibliography (Beley 1985, Bush-Brown 1958, Clarke 1982, Gault 1976, Hints 1975, Kessel 1981, Scott 1984, Taylor 1961). As noted in the Bibliography, rhododendrons can be propagated from seed, by layering, or from cuttings. Special procedures and experience are required to produce new plants from seed, but the use of seed is required to develop new hybrids. Propagation by layering is most easily done by covering a low limb with soil and then cutting the limb from the parent plant after roots are formed. When limbs are not close to the ground, air layering can be done by placing a rooting medium in contact with a limb where roots are wanted and covering the rooting medium with a plastic enclosure. Propagation from a cutting is done by clipping the end of a limb from a plant and placing the cutting in a rooting medium until roots are developed. A plant produced from a cutting will be the same as the parent plant, and this method is the best when a large number of new plants is wanted. However, research and experience are required to successfully propagate rhododendrons from cuttings. Some of the conditions believed to effect the ability to propagate rhododendrons from cuttings are as follows (Beley 1985, Bush-Brown 1958, Clarke 1982, Gault 1976, Hints 1975, Taylor 1961):
1. Rooting medium — Those recommended cover a range of combinations of perlite, sand, and peat moss.
2. Rooting bed covering — Cuttings must be covered while being rooted to prevent an excessive loss of moisture from the leaves.
3. Watering procedure — Recommended methods of watering a rooting bed range from a water mist to a sprinkler system.
4. Maturity of cuttings — Cuttings can be taken from current-year growth or prior-year growth.
5. Type of bud at the end of the cutting — A cutting will have either a new-growth bud or a bloom bud at its end.
6. Type of rhododendron — Some rhododendron plants are reported to be significantly more difficult to propagate than others.
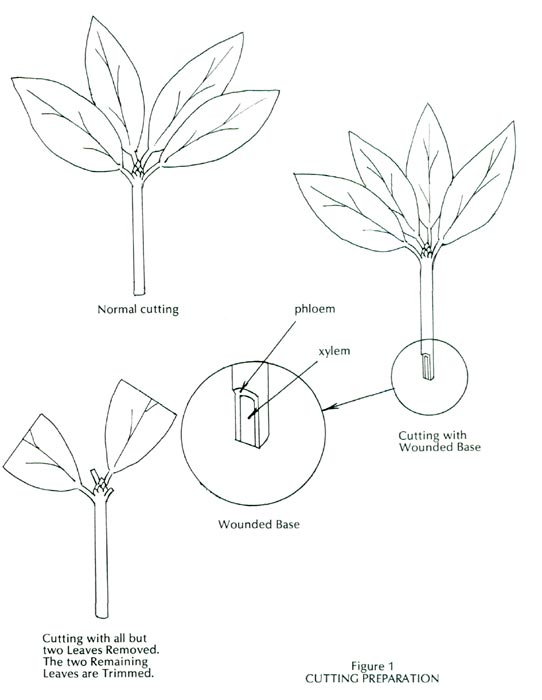
7. Removal of cutting leaves — It has been recommended that all but two or three of the leaves on a cutting be removed to reduce the amount of leaf surface area.
8. Trimming cutting leaves — It has been recommended that the cutting leaves be trimmed in half, as shown by Figure 1, to improve the balance between the amount of leaf area on the cutting and the cutting root system, which is undeveloped.
9. Wounding the base of the cutting — Cutting roots often originate in the cambium area of a cutting. The cambium area is located between the xylem, or woody interior, and the phloem, or exterior bark. If a one-inch slice of bark is removed on one side of the cutting at the base, as shown by Figure 1, the amount of exposed cambium area will be increased and there will be more cambium area to produce roots.
10. Use of a rooting hormone — Hormones are often used when rooting plants from cuttings. The most commonly used rooting hormones are indolebutyric acid, indoleacetic acid, and naphylacetic acid.
11. Cutting turgidity — Cuttings taken in the morning after a soaking rain are generally considered to have a better opportunity to survive than those taken after a period of hot and dry weather.
|
In 1987 a factorial design procedure was used to experimentally determine the effects that trimmed leaves, a wounded base, the use of a rooting hormone, and turgid cuttings had upon the ability to root Rhododendron 'Roseum Elegans' from cuttings. The main effects of the four factors upon the survival of the cuttings were determined to be as follows (Harrington 1987):
Trimmed leaves +22.5%
Wounded base +12.5%
Rooting hormone -2.5%
Turgid cuttings +20%
The small adverse effect that resulted from the use of the rooting hormone was unexpected. The rooting hormone used had the trade name of Rootone, which had active ingredients of 0.2% 1-naphthaleneacetamide, 0.1% indole-3-butyric acid, and 4.04% thiram. As recommended by Hall Nursery, the rooting hormone was applied by mixing 2 teaspoons in a cup of water and soaking the cuttings in the hormone solution overnight before putting them in the rooting bed (Hints 1975). However, the rooting hormone manufacturer recommended wetting the bases of the cuttings, dipping them in the rooting hormone powder, and putting the cuttings into the rooting bed with care taken to make sure that the rooting hormone was not brushed off. To obtain more information concerning the effect of using Rootone as a rooting hormone, further experiments were conducted in 1988 to experimentally determine the results obtained by applying Rootone by both dipping and soaking (Harrington 1988).
The 1988 experiments showed that somewhat better results were obtained when the Rootone was applied by dipping instead of soaking; however, the improvement was small. The effect of applying Rootone by dipping was determined to be a 2.5% improvement in cutting survival (Harrington 1988).
The additional investigations conducted in 1988 using Rootone as the rooting hormone confirmed the poor results that the use of Rootone provides; however, more investigations were required to determine the effects of using other types of rooting hormones. Also, the earlier experiments were conducted with Rhododendron 'Roseum Elegans', and the applicability of the conclusions drawn to other varieties was not known.
The purposes of this experiment were to evaluate the results obtained when using rooting hormones in addition to Rootone and to determine the ability to root rhododendron varieties in addition to R. 'Roseum Elegans'.
Methods and Materials
In order to ensure that the data were repeatable and consistent with the data taken during earlier experiments (Harrington 1987, Harrington 1988), the following conditions, which duplicate those used previously, were maintained:
1. The rooting medium was one half sand and one half peat moss.
2. Cuttings were taken from hardened current-year growth.
3. No cuttings were taken that had terminal bloom buds.
4. The cutting leaves were positioned perpendicular to the average direction of the sun.
5. The rooting bed was covered with a sheet of clear plastic.
6. The rooting bed was watered by sprinkling once per week.
Other conditions of the experiment, which were established by taking advantage of experience gained from earlier experiments (Harrington 1987, Harrington 1988), were as follows:
1. Turgid cuttings were used. Cuttings taken in the morning following a soaking rain resulted in a 20% improvement in survival in one experiment (Harrington 1987) and resulted in a 12.5% improvement in another experiment where other conditions were more favorable (Harrington 1988).
2. All but two cutting leaves were removed. The use of two instead of three cutting leaves has been determined to provide a 20.0% improvement in cutting survival (Harrington 1988).
3. The cutting leaves were trimmed. Trimming the leaves of cuttings having two leaves has been determined to provide a 2.5% improvement in cutting survival (Harrington 1988). Another benefit of trimming the leaves is to reduce the overlapping of leaves in the rooting bed.
4. The cutting bases were not wounded. Wounding the cutting bases can be beneficial using some procedures (Harrington 1987); however, wounding the bases resulted in a 2.5% adverse effect under the conditions of the current experiment (Harrington 1988).
Three varieties of rhododendrons were selected for the experiment. Rhododendron 'Roseum Elegans' was used during earlier experiments (Harrington 1987, Harrington 1988); therefore, it was selected again to provide data that could be compared with data from earlier experiments. Rhododendron 'Catawbiense Album', a large rhododendron that has clusters of large blush-white blooms, was also selected. The third selection was R. euchaites , a large rhododendron that has clusters of large crimson-scarlet blooms.
Four different rooting hormones were selected for evaluation. The commercial product Rootone was used to provide data that could be compared with experiments that had been conducted previously (Harrington 1987, Harrington 1988). The other rooting hormones selected were those that are most commonly recommended for rhododendron propagation (Kessel 1981); they were: indole-3-acetic acid in a powder form, indole-3-butyric acid in a powder form, and naphthalene acetic acid in a paste form.
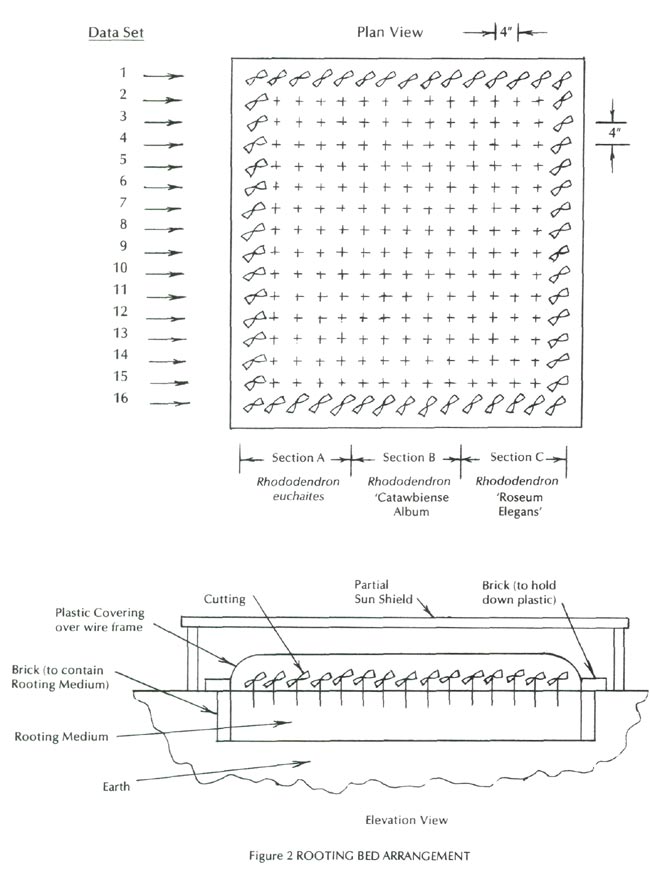
A rooting bed for the cuttings was prepared on July 1,1988 as illustrated by Figure 2. A wire frame to support the plastic covering for the bed was prepared from old fence wire, and a sheet of clear plastic was cut to fit the rooting bed. The plastic covering was put in place with the edges of the plastic held down by bricks to keep the bed from becoming dry. A partial sun shield was made from used boards and put over the rooting bed. The only materials that had to be purchased were the peat moss and the rooting hormones.
|
The strategy used in this project was based upon a four-factor factorial design procedure (Hunter undated). Table 1 outlines the plan for applying the rooting hormones in accordance with a four-factor factorial design procedure. Sixteen data sets were required for a four-factor factorial design procedure, and they corresponded to the sixteen data sets indicated by Table 1. Each of the sixteen data sets outlined in Table 1 consisted of fifteen cuttings, which means that a total of 240 cuttings was required.
| Table 1 The Application of Four Rooting Hormones in Accordance with a Four-factor Factorial Design Procedure | ||||
| Data Set |
lndole-3-
Acetic Acid |
lndole-3-
Butyric Acid |
Rootone | Naphthalene Acetic Acid |
| 1 | 0 | 0 | 0 | 0 |
| 2 | + | 0 | 0 | 0 |
| 3 | 0 | + | 0 | 0 |
| 4 | + | + | 0 | 0 |
| 5 | 0 | 0 | + | 0 |
| 6 | + | 0 | + | 0 |
| 7 | 0 | + | + | 0 |
| 8 | + | + | + | 0 |
| 9 | 0 | 0 | 0 | + |
| 10 | + | 0 | 0 | + |
| 11 | 0 | + | 0 | + |
| 12 | + | + | 0 | + |
| 13 | 0 | 0 | + | + |
| 14 | + | 0 | + | + |
| 15 | 0 | + | + | + |
| 16 | + | + | + | + |
| Key: | 0 = not used | |||
| + = used | ||||
As indicated by Figure 2, the fifteen cuttings in each data set consisted of five each of R. euchaites , R. 'Catawbiense Album', and R. 'Roseum Elegans'. This arrangement permitted the effect of the rooting hormones to be determined for each of the three rhododendrons to evaluate any differences in the effects on the three and also to be averaged for all three.
By following the procedure outlined by Table 1, consecutive pairs of data sets had the same conditions except for the application of indole-3-acetic acid; therefore, the main effect of indole-3-acetic acid was calculated by subtracting the odd data sets from the even data sets and dividing by 8. The main effect of indole-3-butyric acid was found by adding alternate groupings of two data sets, subtracting the first total from the second total, and dividing by 8. The main effect of Rootone was the average difference between alternate groupings of four data sets. And finally, the main effect of naphthalene acetic acid was found by subtracting the first eight data sets from the second eight data sets and dividing by 8.
The main effects of the four factors were readily calculated by applying the appropriate sign convention of the data set values as indicated by Table 3 (Hunter undated). In addition the two-factor interaction effects were also determined by applying the sign convention shown by Table 3 (Hunter undated).
A two-factor interaction effect is the effect upon the results obtained with one factor due to the presence of a second factor. The two-factor interaction effects disclose any effects on the results that are obtained by using the factors in combinations of two. Three-factor interaction effects can also be calculated, but they are generally of little practical significance (Hunter undated).
One situation that could have complicated the significance of the two-factor interaction effects was the fact that the naphthalene acetic acid was available in a lanolin-paste form only whereas the other hormones were available in a powder form. Any of the hormones that were in a powder form were unavoidably applied in a lanolin-paste form when used in combination with the naphthalene acid. Therefore, this fact had to be considered when the two-factor interaction effects that involved naphthalene acid were evaluated.
There was a slow soaking rain on July 21, 1989; therefore, during the morning of July 22, 1989, eighty cuttings were taken from R. euchaites , R. 'Catawbiense Album', and R. 'Roseum Elegans'. Hardened new growth was taken, and no cuttings were taken that had formed bloom buds. All but two leaves were removed from the cuttings, and the two remaining leaves were trimmed as shown by Figure 1.
Each of the sixteen data sets included five cuttings from the three rhododendron varieties as shown by Figure 2.
As indicated by Table 1, the fifteen cuttings that formed data set 1 were placed in the rooting bed with no rooting hormones applied. Only indole-3-acetic acid was applied to the cuttings in data set 2, and only indole-3-butyric acid was applied to data set 3. Both indole-3-acetic acid and indole-3-butyric acid were applied to data set 4.
The procedure outlined by Table 1 was followed for all data sets, with the final data set 16 being given an application of all four rooting hormones.
The indole-3-acetic acid, indole-3-butyric acid, and Rootone were in a powder form; therefore, they were applied by moistening the base of the cuttings and dipping them into the powder. The naphthalene acetic acid was in paste form, and was applied directly to the cutting bases. Care was taken to be sure that the rooting hormones were not wiped off when the cuttings were put into the rooting medium. The rooting bed was watered once every week. The condition of the cuttings was observed every week, and the number of living cuttings of each variety in each data set was recorded as summarized by Table 2. Table 2 shows that all of the cuttings remained alive for the first two weeks; but many of the cuttings died later. By September 23, 1989 very few additional cuttings were dying, and the experiment was concluded on October 14, 1989.
| Table 2 Number of Living Cuttings in each Data Set as Recorded During Weekly Observations | |||||||||||||
| Data Set | Date of Observation | ||||||||||||
| 7-22 | 7-29 | 8-5 | 8-12 | 8-19 | 8-26 | 9-2 | 9-9 | 9-16 | 9-23 | 9-30 | 10-7 | 10-14 | |
| 1A* | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| B** | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| C*** | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 2A | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| B | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| C | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 4 | 4 | 4 | 4 | 4 |
| 3A | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| B | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| C | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 4A | 5 | 5 | 4 | 4 | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| B | 5 | 5 | 5 | 5 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| C | 5 | 5 | 5 | 5 | 4 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 5A | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| B | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| C | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 6A | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| B | 5 | 5 | 5 | 5 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| C | 5 | 5 | 5 | 5 | 5 | 4 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 7A | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| B | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 3 | 3 | 3 | 3 | 3 | 3 |
| C | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 8A | 5 | 5 | 5 | 5 | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| B | 5 | 5 | 5 | 5 | 5 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| C | 5 | 5 | 5 | 4 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 9A | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| B | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| C | 5 | 5 | 5 | 5 | 5 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| 10A | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| B | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| C | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 11A | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| B | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| C | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 12A | 5 | 5 | 5 | 5 | 5 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| B | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| C | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 4 | 4 | 4 | 4 |
| 13A | 5 | 5 | 5 | 5 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| B | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| C | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 14A | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| B | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| C | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 15A | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| B | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| C | 5 | 5 | 5 | 5 | 4 | 4 | 4 | 4 | 3 | 3 | 3 | 3 | 3 |
| 16A | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 4 | 4 | 4 | 4 |
| B | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| C | 5 | 5 | 5 | 5 | 5 | 4 | 3 | 3 | 3 | 2 | 2 | 2 | 2 |
| *A R. euchaites | |||||||||||||
| **B R. 'Catawbiense Album' | |||||||||||||
| ***C R. 'Roseum Elegans' | |||||||||||||
Results
The numbers of living cuttings of each variety in each data set when the experiment ended are shown in Table 2 and are carried forward to the first column of Table 3 in percentage form. As shown by Table 3, the average cutting survival rates for
R. euchaites
, R. 'Catawbiense Album', and R. 'Roseum Elegans' were 90.0%, 86.3%, and 78.8%, respectively. The grand average survival rate for all varieties was 85.0%.
As discussed previously, the main effects and the two-factor interaction effects were easily determined by calculating the average difference between select pairs of the sixteen data sets. The calculations were simplified by assigning the algebraic signs indicated on Table 3 to the respective data set values, summing the resulting data set values, and dividing by 8 (Hunter undated). The basis for the algebraic signs shown by Table 3 for the main effects can be understood by referring to Table 1, where it may be seen that a plus sign indicates that a factor is present.
Table 3 shows that the average main effects of the four rooting hormones tested upon the survival of rhododendron cuttings were as follows: indole-3-aceticacid -18.3%, indole-3-butyricacid -13.3%, Rootone -10.0%,
naphthaleneaceticacid +11.7%.
Table 3 also shows that the most significant two-factor interaction effect was +16.7% and took place between indole-3-acetic acid and naphthalene acetic acid. Naphthalene acetic acid had a +1.7% two-factor interaction effect with both indole-3-butyric acid and Rootone. Rootone had a two-factor interaction effect of zero with indole-3-butyric acid and -1.7% with indole-3-acetic acid. The most unfavorable two-factor interaction effect was -8.3% and was between indole-3-acetic acid and indole-3-butyric acid.
| Table 3 Experimental Determination of the Main Effects and the Two-Factor Interaction Effects that Four Rooting Hormones have on the Ability to Propagate Rhododendrons from Cuttings | ||||||||||||
| Two-Factor Interaction Effects | ||||||||||||
| Main Effects | Indole-3-Acetic Acid and Indole-3-Butyric Acid | Indole-3-Acetic Acid and Rootone | Indole-3-Acetic Acid and Naphthalene Acetic Acid | Indole-3-Butyric Acid and Rootone | Indole-3-Butyric Acid and Naphthalene Acetic Acid | Rootone and Naphthalene Acetic Acid | ||||||
| Data Set |
Data Set Value
% |
Average Data Set Value
% |
Indole-3-Acetic Acid | Indole-3-Butyric Acid | Rootone | Naphthalene Acetic Acid | ||||||
| 1A* | 100 | 100 | -- | -- | -- | -- | + | + | + | + | + | + |
| B** | 100 | |||||||||||
| C*** | 100 | |||||||||||
| 2A | 100 | 93.3 | + | -- | -- | -- | -- | -- | -- | + | + | + |
| B | 100 | |||||||||||
| C | 80 | |||||||||||
| 3A | 100 | 100 | -- | + | -- | -- | -- | + | + | -- | -- | + |
| B | 100 | |||||||||||
| C | 100 | |||||||||||
| 4A | 60 | 46.7 | + | + | -- | -- | + | -- | -- | -- | -- | + |
| B | 40 | |||||||||||
| C | 40 | |||||||||||
| 5A | 100 | 100 | -- | -- | + | -- | + | -- | + | -- | + | -- |
| B | 100 | |||||||||||
| C | 100 | |||||||||||
| 6A | 100 | 53.3 | + | -- | + | -- | -- | + | -- | -- | + | -- |
| B | 20 | |||||||||||
| C | 40 | |||||||||||
| 7A | 100 | 86.7 | -- | + | + | -- | -- | -- | + | + | -- | -- |
| B | 60 | |||||||||||
| C | 100 | |||||||||||
| 8A | 60 | 53.3 | + | + | + | -- | + | + | -- | + | -- | -- |
| B | 60 | |||||||||||
| C | 40 | |||||||||||
| 9A | 100 | 93.3 | -- | -- | -- | + | + | + | -- | + | -- | -- |
| B | 100 | |||||||||||
| C | 80 | |||||||||||
| 10A | 100 | 100 | + | -- | -- | + | -- | -- | + | + | -- | -- |
| B | 100 | |||||||||||
| C | 100 | |||||||||||
| 11A | 100 | 100 | -- | + | -- | + | -- | + | -- | -- | + | -- |
| B | 100 | |||||||||||
| C | 100 | |||||||||||
| 12A | 80 | 86.7 | + | + | -- | + | + | -- | + | -- | + | -- |
| B | 100 | |||||||||||
| C | 80 | |||||||||||
| 13A | 80 | 93.3 | -- | -- | + | + | + | -- | -- | -- | -- | + |
| B | 100 | |||||||||||
| C | 100 | |||||||||||
| 14A | 100 | 100 | + | -- | + | + | -- | + | + | -- | -- | + |
| B | 100 | |||||||||||
| C | 100 | |||||||||||
| 15A | 80 | 80 | -- | + | + | + | -- | -- | -- | + | + | + |
| B | 100 | |||||||||||
| C | 60 | |||||||||||
| 16A | 80 | 73.3 | + | + | + | + | + | + | + | + | + | + |
| B | 100 | |||||||||||
| C | 40 | |||||||||||
| Avg. | ||||||||||||
| A | 90.0% | -10.0% | -15.0% | - 5.0% | 0% | -15.0% | +5.0% | +10.0% | 0% | +5.0% | -5.0% | |
| B | 86.3 | -17.5 | -7.5 | -12.5 | +27.5 | +2.5 | -2.5 | +17.5 | +7.5 | +7.5 | +12.5 | |
| C | 78.8 | -27.5 | -17.5 | -12.5 | +7.5 | -12.5 | -7.5 | +22.5 | -7.5 | -7.5 | -2.5 | |
| Grand Avg. | 85.0% | -18.3% | -13.3% | -10.0% | +11.7% | -8.3% | -1.7% | +16.7% | 0% | +1.7% | +1.7% | |
| *A R. euchaites | ||||||||||||
| **B R. 'Catawbiense Album' | ||||||||||||
| ***C R. 'Roseum Elegans' | ||||||||||||
Conclusions and Recommendations
The experiments conducted in 1989 confirmed the validity of the conclusions reached during similar experiments in 1987 and 1988 and provided further insight concerning rhododendron propagation (Harrington 1987, Harrington 1988).
Experiments were conducted with R. euchaites (red), R. 'Catawbiense Album' (white), and R. 'Roseum Elegans' (pink) which have distinctly different characteristics. The survival rate ranged from 78.8% (R. 'Roseum Elegans') to 90.0% ( R. euchaites ) with a grand average of 85.0%. The length restrictions on this report prohibited an analysis of the statistical significance of the data; however, the experiments included eighty specimens from each rhododendron; consequently, there is confidence in the conclusion that the ability to propagate the three rhododendrons is approximately the same under the conditions tested.
The results shown by Table 3 indicate that the main effects upon the three rhododendrons are generally consistent. The most unusual main effect on any rhododendron was the +27.5% main effect of naphthalene acetic acid on R. 'Catawbiense Album'.
The most favorable main effect was obtained with naphthalene acetic acid. The average main effect was +11.7%, and it is noted that a positive effect of this magnitude is of great significance. The baseline conditions outlined earlier for this experiment are very favorable for the propagation of rhododendrons. Referring to Table 3, it can be seen that a grand average of 85.0% of all cuttings survived, and that average included the net adverse effect of the rooting hormones. Since the survival cannot exceed 100%, the magnitude of the beneficial effects that can be achieved is limited. It is, therefore, concluded that the +11.7% main effect of naphthalene acetic acid is important.
The -10.0% main effect of Rootone compares with effects of -2.5% in 1987 and +2.5% 1988, both of which were obtained under less favorable test conditions (Harrington 1987, Harrington 1988). The response of all varieties to Rootone was a consistent adverse effect.
Indole-3-butyric acid, which is one of the active ingredients in Rootone, had an average main effect of -13.3%. Again, there was a consistent adverse effect on all varieties.
The most adverse main effect was caused by indole-3-acetic acid. There was a -18.3% average main effect, and there was an adverse main effect on all three varieties.
The most favorable two-factor interaction effect was +16.7%, which was between indole-3-acetic acid and naphthalene acetic acid. However, the apparent advantage of using these two hormones in combination would be more than offset by the -18.3% adverse main effect of indole-3-acetic acid.
Indole-3-butyric acid and naphthalene acetic acid are active ingredients of Rootone, and the two-factor interaction effects of these two acids with Rootone are zero and +1.7%, respectively. These effects are negligibly small as expected.
Negligibly small two-factor interaction effects of -1.7% and +1.7% were also recorded between indole-3-acetic acid and Rootone and between indole-3-butyric acid and naphthalene acetic acid, respectively.
The most adverse two-factor interaction effect was between indole-3-acetic acid and indole-3-butyric acid and was -8.3%. The base conditions for the 1989 experiments, which were defined earlier, were established by taking advantage of the results from earlier experiments (Harrington 1987, Harrington 1988). Under these highly favorable conditions for propagating rhododendrons, the use of indole-3-acetic acid, indole-3-butyric acid, and Rootone were determined to provide adverse results when used as rooting hormones. Naphthalene acetic acid was found to provide highly beneficial results when used as a rooting hormone. The 1989 experiments also demonstrated that the rhododendrons R. euchaites , R. 'Catawbiense Album', and R. 'Roseum Elegans', which have characteristics that are significantly different, can be propagated from cuttings with a high probability of success. Additional experiments are recommended to broaden our knowledge of rhododendron propagation. A more extensive investigation concerning the use of naphthalene acetic acid as a rooting hormone would be of great interest. Also, experiments with additional rhododendrons, such as the unique hybrids, would be informative.
Bibliography
Beley, Jim (ed.). All About Azaleas, Camellias & Rhododendrons. San Francisco: Chevron Chemical Co., 1985.
Bush-Brown, James and Louise. America's Garden Book. New York: Charles Scribner's Sons, 1958.
Clarke, Harold J. Getting Started With Rhododendrons and Azaleas. Portland, Oregon: The American Horticultural Society, 1982.
Cault, S. Millar. The Color Dictionary of Shrubs. New York: Crown Publishers, 1976.
Harrington, John Lee. "Rhododendron Propagation from Cuttings". VJAS Science Fair Project Report. Menchville High School. Newport News, Virginia, 1987.
Harrington, John Lee, "Further Experimentation Concerning Rhododendron Propagation." VJAS Science Fair Project Report. Menchville High School. Newport News, Virginia, 1988.
"Hints on Growing Rhododendrons". Bridgewater, Mass: Hall Nursery, 1975.
Hunter, J.S. "Some Simple Factorial Designs and Their Application". Princeton, New Jersey: Princeton University, (undated).
Kessell, Mervyn S. Rhododendrons and Azaleas. Dorset, England: Blandford Books Ltd., 1981.
Scott, Jane. Botany in the Field. Englewood Cliffs, New Jersey: Prentice-Hall Inc., 1984.
Taylor, Norman. Taylor's Encyclopedia of Gardening . Cambridge, Mass.: The Riverside Press, 1961.
John Harrington is a Junior at Menchville High School in Newport News, Virginia. The research project that this article is based upon won first prize in the Botany Division at the 1989 Virginia Junior Academy of Science Fair. John is an Eagle Scout and met Dr. Martha Roane at the 1989 National Boy Scouts of America Jamboree. Dr. Roane encouraged him to submit this article to the ARS Journal.
John intends to do further studies concerning rhododendron propagation using naphthalene acetic acid as a rooting hormone. He would like to work with other rhododendrons, if he can find a source for cuttings and is also interested in investigating wounding the cutting base with a slit rather than carving away a slice.
