JARS v44n3 - Doubling Chromosomes with Colchicine Treatment in Vitro as Determined by Chloroplast Number in Epidermal Guard Cells
Doubling Chromosomes with Colchicine Treatment in Vitro as Determined by Chloroplast Number in Epidermal Guard Cells
Donald W. Paden, Martin M. Meyer, Jr., A. Lane Rayburn
University of Illinois Urbana-Champaign, Illinois
Over the years a number of articles concerning the doubling of chromosomes in certain rhododendron have appeared in the Quarterly Bulletin of the American Rhododendron Society (Kehr, 1966; 1971; 1973; 1975; Jones, 1977). Those who attempt this transformation hope to produce plants with larger flowers, better foliage and improved general substance (Pryor & Frazier, 1968). More importantly, a new gene pool becomes available for propagation purposes (Kehr, 1966; Jones, 1977). In 1985 we received a modest grant from the ARS Research Foundation for the study of in vitro colchicine treatment of rhododendron. The objective was to adapt colchicine doubling techniques to the culture of rhododendron in vitro. (In vitro refers to propagation "in glass" under sterile conditions.) As the study has progressed, perhaps of equal or greater importance for members of the Society has been the use of chloroplast observation in guard cells for determining whether the number of chromosomes has, in fact, been doubled.

|
The procedures were importantly influenced by articles on in vitro colchicine treatment of blueberries (Lyrene & Perry, 1982; Goldy & Lyrene, 1984). In the experiment with blueberries, Anderson's Rhododendron Medium (ARM) was used. After some trial and error it was determined that for this variety of blueberries the best colchicine strength was 0.025 percent. The in vitro blueberry cuttings were placed in the dark for 7 days, returned to light for from 48 to 96 hours, placed on a rotating wheel with liquid ARM containing colchicine for 24 to 48 hours, rinsed, and returned to solid medium (see also, Pryor & Frazier, 1968).
The experiment was carried on in part at the University of Illinois in the tissue culture laboratory of the Department of Horticulture (the recipient of the grant) and in part in the kitchen and basement of the Paden household. The treatment of the rhododendron cultures between the two sites differed somewhat with respect to timing, treatment, care of the cultures and subsequent handling of the plants.
Procedures and Preliminary Observations
Colchicine treatment of plants is relatively easy, since it is soluble in water at the rate of 1 gram in 22 milliliters (ml) of water. At the University laboratory, 1.6 grams were dissolved in 100 ml of water, filter sterilized, and used as a stock solution. Solutions with concentrations of .002%, .004%, 0.02%, and 0.04% were then prepared from the stock solution for use in the experiment. In vitro cultures of actively dividing shoots of 'White Felicity' ([(
R. carolinianum
x
R. dauricum
) x
R. dauricum
] by Paden), 'K. D. Harris', 'Nova Zembla' and 'Wendy Lyn' (['Princess Elizabeth' x (
R. smirnowii
x
R. yakushimanum
)] by Delp) were transferred to 4 ml of the liquid medium in each of these four concentrations in test tubes which were rotated at one RPM for 48 hours. The cultures were then removed from the liquid medium, rinsed, and placed on fresh proliferation medium with agar but without colchicine.
There was some varietal difference in injury and reduced growth caused by the colchicine treatment. 'Nova Zembla' seemed the most sensitive and 'White Felicity' the least, with the other cultivars in this experiment in between. The cultures were allowed to continue to form shoots which were eventually placed in a rooting medium. These shoots were then transferred to soil for further growth.
An attempt was made to replicate the blueberry procedure with rhododendron in the home laboratory, although under somewhat primitive conditions and without a rotating wheel to constantly bathe the cuttings with the colchicine solution. Rather, the in vitro cuttings were manually shaken in the solution as frequently as could be arranged (4 or 5 times an hour except at night). Although sanitary conditions for the culture in the home laboratory were undoubtedly not as good as those at the University laboratory, contamination was not great and the procedure successful. When the shoots were transferred to soil, they were systematically graded and those with stems of the largest diameter were rooted separately.
Fairly mature plants from both laboratories were used in judging the outcome of the treatments: 'Wendy Lyn' - 20 plants, 'K.D. Harris' - 10 plants, 'Nova Zembla' - 10 plants, 'White Felicity' - 20 plants
Ploidy Determination:
The preferred method of ploidy determination in plant species is chromosome counting. The small chromosomes of the rhododendron, however, as well as the limited amount of tissue containing dividing (observable) chromosomes make counting chromosomes difficult. In determining ploidy levels for large numbers of plants this difficulty becomes overwhelming. Kehr (1971) used several characteristics to determine ploidy level in rhododendron. He noted that epidermal guard cells were larger in tetraploid species than in diploid species. In other plant species, the large guard cells of tetraploids have been observed to contain more chloroplasts than the diploid guard cells (Dudley, 1958). This observation also holds true in corn:
zea maize
(Rayburn, unpublished). Due to the relative ease of the procedure, we decided to try this with rhododendron.
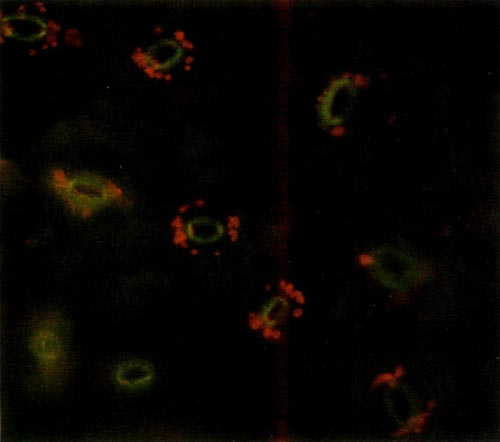
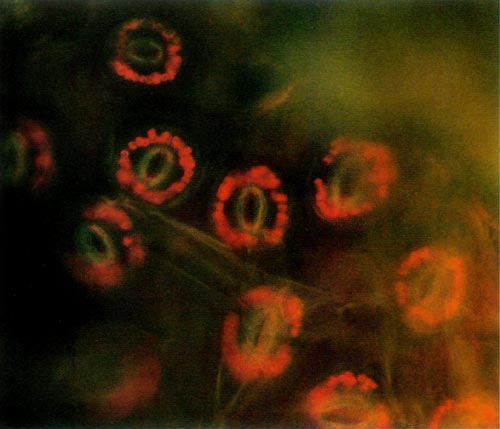
The procedure requires access to an epi-fluorescence microscope. This instrument allows one to expose a specimen to one particular wavelength of light. Due to particular characteristics of various molecules, different substances absorb light of different wavelengths. Upon absorbing the energy the molecules become excited and are raised to a higher state. When the molecules return to their lower state, light energy is given off. This is called fluorescence. Chlorophyll, the principle pigment in chloroplast, is excited by light of 430 nanometers (nm) and emission of the fluorescence is at 669nm. The chloroplast absorbs blue light and appears red when viewed under the fluorescent microscope. The color photos (Figures 1 and 2) reproduce almost precisely what the viewer sees using the microscope.
|
|
Figure 1. Epidermal guard cells of a diploid 'Wendy Lyn'
Photo by A. Lane Rayburn |
|
|
Figure 2. Epidermal guard cells of a tetraploid 'Wendy Lyn'
Photo by A. Lane Rayburn |
The epidermis of mature leaves is the tissue of choice. The lower epidermis is peeled from the leaf and a small section is placed on a microscope slide. Distilled water is then placed on the epidermis, and a cover-slip is applied and flattened with slight pressure. The slide is then placed on the microscope and observed. The oval yellow structure consists of two cells that are seen to have the classical anatomy of dicot guard cells. The small red circular objects surrounding these cells are the chloroplasts. Thus, each stomate consists of two guard cells with accompanying chloroplasts and other appropriate cell structures. The diploid chloroplast number of an untreated plant was about half that of a known tetraploid plant. Thus, a leaf of a normal diploid R. carolinianum supplied by Kehr had 6-8 chloroplasts per ceil while a leaf of 'Epoch' (tetraploid R. carolinianum ) from Kehr was observed to have 14 chloroplasts. A leaf supplied by Delp of 'Small Fry' ('Epoch' selfed) had 16-18 chloroplasts per cell.
Counting the chloroplast proved to be relatively easy. An examination of leaves of 'Arctic Pearl' and 'Power House' (from Delp) revealed that only the latter might be doubled (it being impossible to reach a definite conclusion about this without having examined the leaves of the plant's parents). Leaves of 'Lodestar' x R. yakushimanum and 'Professor Amateis' (both treated with colchicine in vitro) were apparently unaffected by the treatment. Photographs of two of the untreated cultivars are shown in Figures 3 and 4.

|

|
|
|
Figure 3. Untreated 'White Felicity'
Photo by Donald Paden |
Figure 4. Untreated 'Wendy Lyn'
Photo by Weldon Delp |
Experimental Results
Having affirmed the relationship between chloroplast and ploidy levels for rhododendron, the experimental plants were next examined. Potential tetraploid plants of both 'White Felicity' and 'Wendy Lyn' were compared to normal plants. A number of the plants of 'Wendy Lyn' had twice the chloroplast number per guard cell as the diploid 'Wendy Lyn'. This was also true for the 'White Felicity' plants. Thus, the number of chloroplasts per guard cell would appear to be a rapid and relatively easy method (roughly 10 minutes per plant) to determine ploidy level in these cultivars. The limiting factor for the use of this technique is the availability of an epi-fluorescence microscope. It should be noted that this type of microscope is becoming increasingly available at most colleges and universities.
Eleven of the 20 'Wendy Lyn' plants examined appeared to be doubled, as were two of the 20 'White Felicity'. Approximately 100 very small plants that had resulted from the in vitro colchicine treatment were distributed to members of the Midwest Chapter, ARS, for growing on. A few plants of all of our treated cultivars were included for each interested member. Tom Homer, one of the recipients, observed that a plant of 'White Felicity' looked very different from the rest and returned the plant to us. Both this and a similar unusual looking 'White Felicity' in the Paden garden appeared - from an examination of the chloroplast number - to be tetraploid.

|
|
Figure 5. Treated plants of 'Wendy Lyn':
left, not affected; right, doubled. Photo by Donald Paden |
Plants of 'Wendy Lyn' which seem to have been affected are much smaller than the unaffected plants (Figure 5). Three or four plants of 'Wendy Lyn' that presumably were not treated with colchicine were, however, treated with a growth retardant. Ironically, these grew dense with wavy leaves, and numerous flower buds (Figure 6). One of these plants and the parent plant of all of the 'Wendy Lyn' plants (obviously not doubled) exhibit the same ploidy level.

|
|
Figure 6. Plant of 'Wendy Lyn' treated with a growth
retardant but not treated with colchicine Photo by Donald Paden |
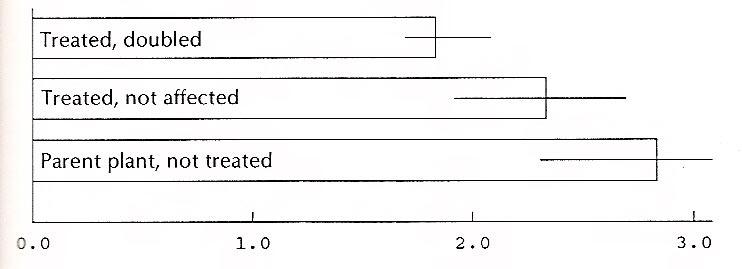
The ratios of the length of the leaves of the 11 'Wendy Lyn' plants affected by colchicine and the 8 that were not doubled were calculated. (The twentieth plant was one of those treated with the growth retardant.) The usual assumption is that plants with doubled chromosomes will have broader leaves; that is, the ratio of the length to the width (l/w) will be smaller. The data below seem to indicate this, although very vaguely. Moreover, the number of leaves (and plants) in each group is small. Since the plants in each group were not a random sample of all plants treated (many had been given away), the data cannot be used to calculate the percent of all the treated plants that were doubled, nor is a calculation of the statistical significance of the difference legitimate.
Figure 7 shows the ratios for three categories of plants of 'Wendy Lyn'. The horizontal lines at the end of each bar in the graph indicate the range between the largest and smallest ratio within each group. The overlap between groups signals caution in drawing conclusions from the data. For 'White Felicity' the ratios for affected and unaffected plants showed no difference. In this case, however, the data are too limited to support a conclusion of any kind.
|
| Figure 7. Ratio of length to width, 'Wendy Lyn' leaves. |
Conclusions
Several observations are worth repeating. First, the counting of chloroplast number in epidermal guard cells proved to be an effective method of determining ploidy levels in rhododendron. Second, different hybrids and species seem to respond differently to colchicine treatment with the result that the use of different strengths of colchicine solutions would be necessary. Third, the use of colchicine in vitro may result in a number of plants whose ploidy levels have been changed and which must be studied to determine good and bad characteristics if, indeed, the plants actually differ one from another. Finally, reliance upon simple characteristics of treated plants (such as the ratio of leaf width to breadth) should be viewed with caution.
In the long run, the characteristics of the flowers and mature plant habit of our experimental plants will need to be studied and reported at some future time.
Acknowledgements
The authors express their appreciation to August Kehr and Weldon Delp for providing leaves, commenting on the manuscript and providing encouragement throughout the research.
References
Dudley, J. W. (1958). Number of chloroplast in the guard cells of inbred lines of tetraploid and diploid sugar beets.
Agronomy Journal
, 50,169-170.
Eigsti, O. J., & Dustin, P., Jr. (1955). Colchicine in Agriculture, Medicine, Biology, and Chemistry . Ames, IA, The Iowa State College Press.
Goldy, R. C., & Lyrene, P. M. (1984). In vitro colchicine treatment of 4 x blueberries, vaccinium sp. Journal of the American Society of HortScience , 109(3), 336-338.
Jones, E. A. (1977). Using colchicine for plant improvement. Quarterly Bulletin of the American Rhododendron Society , 31(4), 220-222.
Kehr, A. E. (1966). Breeding for a purpose. Quarterly Bulletin of the American Rhododendron Society , 20(2), 130-141.
Kehr, A. E. (1971). A tetraploid Rhododendron carolinianum. Quarterly Bulletin of the American Rhododendron Society , 25(1), 4-7.
Kehr, A. E. (1973). Questions and Answers. Quarterly Bulletin of the American Rhododendron Society , 27(1), 20-23.
Kehr, A. E. (1975). Recent additions to published rhododendron numbers. Quarterly Bulletin of the American Rhododendron Society , 29(2), 110-113.
Lyrene, P. M., & Perry, J. L. (1982). Production and selection of blueberry Dolyploids in vitro. The Journal of Heredity , 73, 377-378.
Pryor, R. I. & Frazier, L. C. (1968). Colchicine-induced tetraploid azaleas. Horticultural Science , 3(4), 383-385.
Sax, K. (1930). Chromosome stability in the genus Rhododendron . American Journal of Botany , xvii(4), 247-258.
Dr. Paden, Professor of Economics Emeritus, Dr. Meyer, Professor of Nursery Management, and Dr. Rayburn, Assistant Professor of Cytogenetics, University of Illinois at Urbana-Champaign, report here on research partially funded by the American Rhododendron Society Research Foundation.
