JARS v45n3 - Seed Germination of Rhododendron catawbiense and Rhododendron maximum: Influence of Light and Temperature
Seed Germination of Rhododendron catawbiense and Rhododendron maximum: Influence of Light and Temperature
Frank A. Blazich, Professor
Stuart L. Warren, Assistant Professor
Juan R. Acedo, Research Technician
William M. Reece, Research Technician
Department of Horticultural Science, North Carolina State University
Raleigh, North Carolina
Reprinted from the Journal Environmental Horticulture, Vol. 9, No. 1, March 1991
Abstract
Seeds of
Rhododendron catawbiense
Michx. and
Rhododendron maximum
L. were germinated at 25°C (77°F) and 25°/15°C (77°/59°F) with daily photo-periods of 0, 1/2, 1/2 twice daily, 1, 2, 4, 8, 12, or 24 hr. After 30 days at 25°C (77°F), germination of
R. catawbiense
in darkness was 5% but increased to 64% at 25°/15°C (77°/59°F). At both temperatures, germination > 95% was attained by day 15 for photoperiods of 1/2 to 12 hr. Regardless of temperature, seeds of
R. maximum
required light for germination. At 25°C (77°F), increasing photoperiods increased germination with 79 and 81% germination occurring by day 21 for the 12- and 24-hr, photoperiods, respectively. The alternating temperature of 25°/15°C (77°/59°F) enhanced germination when light was limiting. At this temperature, germination of 92 to 97% was reached by day 21 for photoperiods > 4 hr.
Index words: seeds, sexual propagation, native plants, catawba rhododendron, rosebay rhododendron
Significance to the Nursery Industry
Quantitative data are presented regarding the influence of light and temperatures of 25°C (77°F) and 25°/15°C (77°/59°F) on seed germination of R. catawbiense and R. maximum . Without light, germination of R. catawbiense at 25°C (77°F) was low (5%) although moderate germination (64%) occurred at 25°/15°C (77°/49°F). On the other hand, R. maximum required light for germination regardless of temperature. Therefore, to maximize germination, seeds of each species should be subjected to light. At both temperatures, daily photoperiods of 1/2 to 12 hr. permitted germination of R. catawbiense to exceed 95% by 15 days. For R. maximum , 80% germination resulted by 21 days at 25°C (77°F) with daily photoperiods of 12 and 24 hr. The alternating temperature of 25°/15°C (77°/59°F) enhanced germination when light was limiting. At this temperature, germination approaching 100% was reached by 21 days for photoperiods > 4 hr.
Introduction
Rhododendron catawbiense
(catawba rhododendron) and
Rhododendron maximum
(rosebay rhododendron) are indigenous to North America and are highly prized as landscape plants because of their showy flowers.
Rhododendron catawbiense
occurs only in the United States from West Virginia and Virginia south to Georgia and Alabama (5).
Rhododendron maximum
has a far broader range extending form Ontario, Quebec and Nova Scotia, Canada southward through the New England States, continuing into New York and Pennsylvania along the Appalachian Mountains to Georgia (4). Both species are evergreen and generally grow as shrubs. In recent years, popularity and demand for
R. catawbiense
and
R. maximum
have intensified because of increased interest in native plants. To satisfy demand, many nurserymen utilize sexual propagation.
Many references can be found regarding seed germination of species of Rhododendron . Generally, such references mention that seeds can be germinated and usually describe procedures which have proven successful. Some experts also mention the need of light for germination but lack such critical information as the number of hours required daily and the interaction of light and temperature (1, 2, 7).
Recently, Malek et al. (6) described the relationship between light and temperature in seed germination of the deciduous species, flame azalea ( Rhododendron calendulaceum (Michx.) Torr.]. This was one of the first reports to quantify the relationship between photoperiod and temperature for seed germination of a species of Rhododendron . Similar work is needed for other species, particularly R. catawbiense and R. maximum . No research has been reported on seed germination of R. catawbiense . On the other hand, seeds of R. maximum require light for germination, and the intensity (irradiance) needed for germination is relatively low [i.e. 12 foot-candles (129 lx)] (8). Because little is known about the effects of photoperiod and temperature on germination of these species, the objective of this research was to explore the influence of varying photoperiods and constant versus alternating temperature on seed germination of R. catawbiense and R. maximum .
Materials and Methods
On November 13, 1986 mature seed capsules were collected from native populations of open-pollinated plants of
R. catawbiense
and
R. maximum
growing in western North Carolina. Plants of
R. catawbiense
were located in Buncombe County at an elevation of 1860 m (6100 ft) and the site for
R. maximum
was in Avery County at an elevation of 950 m (3100 ft). Capsules were stored in paper bags at 20°C (68°F) for 30 days. Seeds were then removed from the capsules and stored in sealed bottles at 4°C (39°F). At storage, the moisture content of seeds of
R. catawbiense
and
R. maximum
was 7% and 6%, respectively [approximately 170,000 and 330,000 pure seeds per 28 g (1 oz) for
R. catawbiense
and
R. maximum
, respectively (Fig. 1)]. Moisture content was determined by calculating the mean moisture content of six, 100 seed samples for
R. catawbiense
and six, 200 seed samples for
R. maximum
following drying at 105°C (221 °F) for 24 hr.
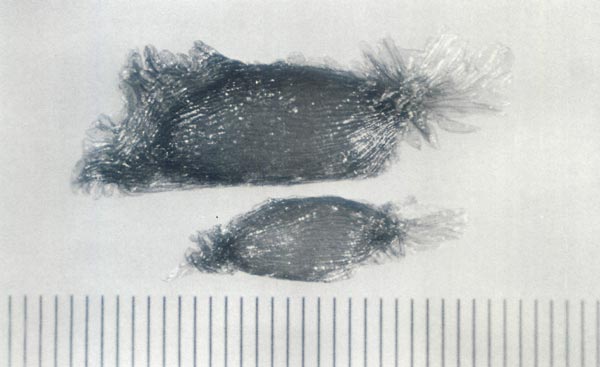
|
|
Fig. 1. Seeds of
Rhododendron catawbiense
(top)
and Rhododendron maximum (bottom). Note the difference in size between the species Scale divisions = 0.1 mm (0.004 in). |
In March 1989, seeds of each species were removed from storage and graded by manual removal of abnormal, damaged and undersized seeds. Graded seeds were sown in covered 9 cm (3.5 in) glass Petri dishes containing two pre-washed germination blotters moistened with tap water. Following placement of seeds in the dishes, half were designated for germination at 25°C (77°F) and the other half for germination at an 8/16 hr. thermo-period of 25°/15°C (77°/59°F). All dishes were placed in black sateen cloth bags and the seeds allowed to imbibe overnight at 21 °C (70°F). The next day, bags were randomized within two growth chambers [C-chambers (3)] set at the appropriate temperatures. Chamber temperatures varied within ± 0.5°C (0.9°F) of the set point.
Within each temperature regime, seeds were subjected daily to the following nine photoperiods: total darkness, 1/2, two 1/2 hr. photoperiods separated by 7½ hr. of darkness, 1, 2, 4, 8, 12, or 24 hr. Regardless of temperature, photoperiod treatments were administered the same time each day. and for the alternating temperature of 25°/15°C (77°/59°F), all photoperiod treatments with the exception of total darkness and 24 hr. began with the transition to the high temperature portion of the cycle. Growth chambers were equipped with cool-white fluorescent lamps which provided a photosynthetic photon flux (400-700 nm) of 40µmol m -2 s -1 (3.0 klx) as measured at dish level with a cosine corrected LI-COR LI-185 quantum/ radiometer/photometer. All photoperiod treatments, except total darkness and the 24 hr. irradiation, were regulated by removal and placement of the Petri dishes in black sateen cloth bags. For the 24 hr. photoperiod treatment, the Petri dishes remained continuously un-bagged in open chamber conditions. Regardless of the photoperiod, temperatures within the Petri dishes as measured with a thermocouple, never exceeded ambient by more than 1°C (2°F). Petri dishes representing the total darkness treatment were kept in the black cloth bags throughout the experiment and all watering and germination counts were performed under a green safelight. Germination blotters were kept moist with tap water throughout the experiment. Seeds showing signs of decay were immediately removed from the dishes. Each photoperiod treatment was replicated four times within each temperature regime, with a replication consisting of a Petri dish containing 100 seeds. Germination counts were recorded every 3 days for 30 days. A seed was considered germinated when the emerging radicle was > 1 mm (0.04 in).
Percent germination was calculated as a mean of four replications per treatment. Within each temperature, data were subjected to analysis of variance procedures and regression analysis (9).
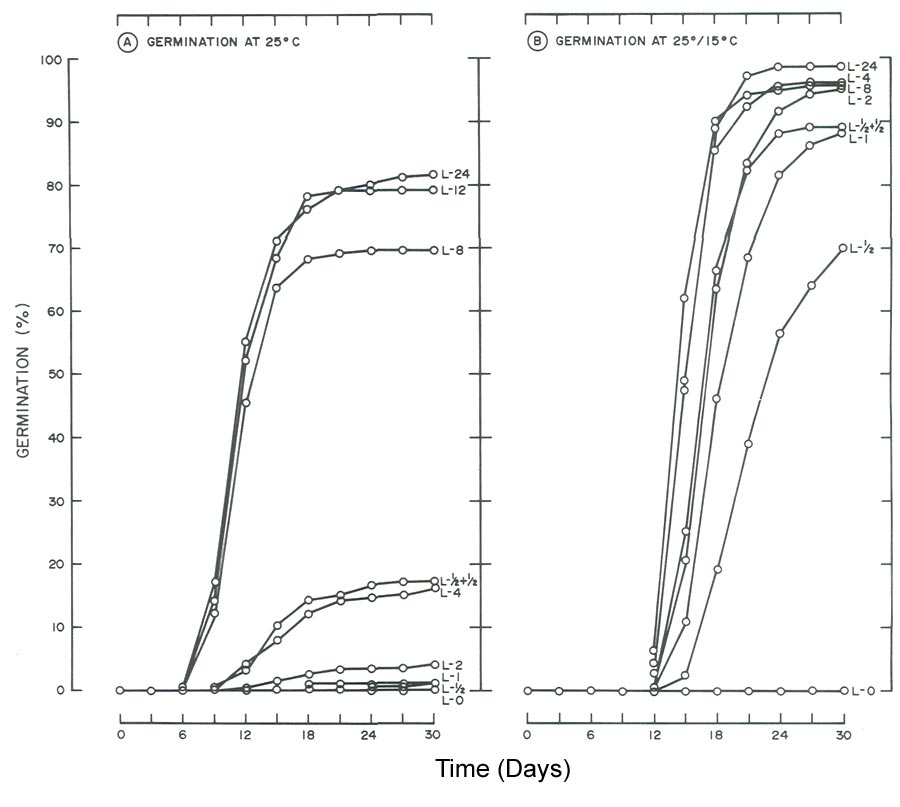
|
|
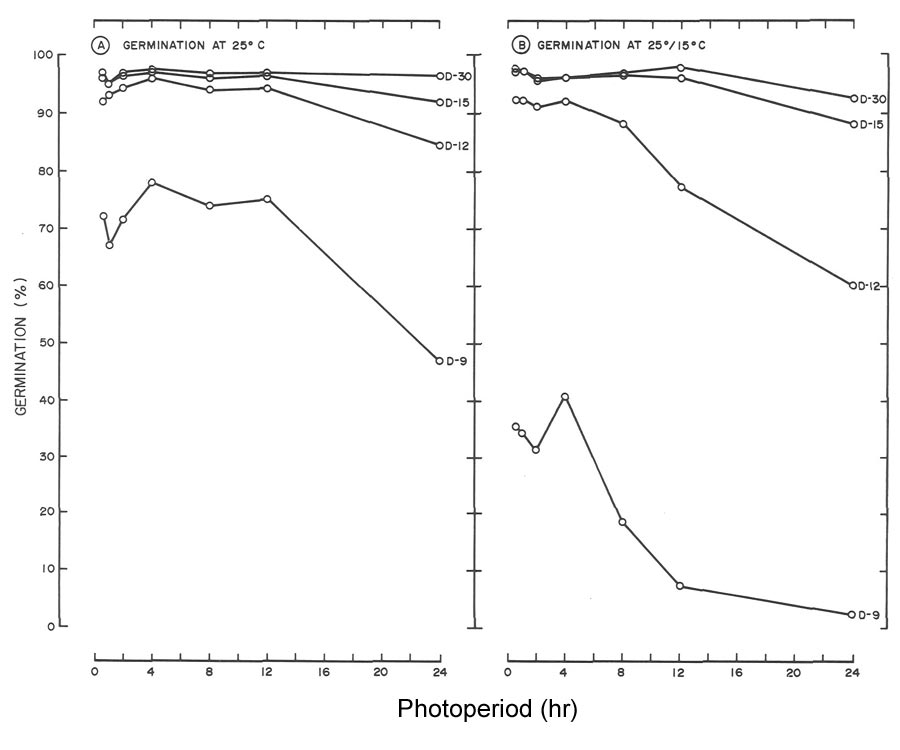
Fig. 2. Influence of light and temperature on seed germination of
R. maximum
. (A) germinated at 25°C (77°F)
with daily photoperiods (L) ranging from total darkness (L-O) to 24 hr. (L-24). (B) germinated at 25°/15°C (77C/59°F) utilizing the same photoperiods as in A. Data for the 12-hr, photoperiod were omitted since germination was similar to the 24-hr, light treatment. |
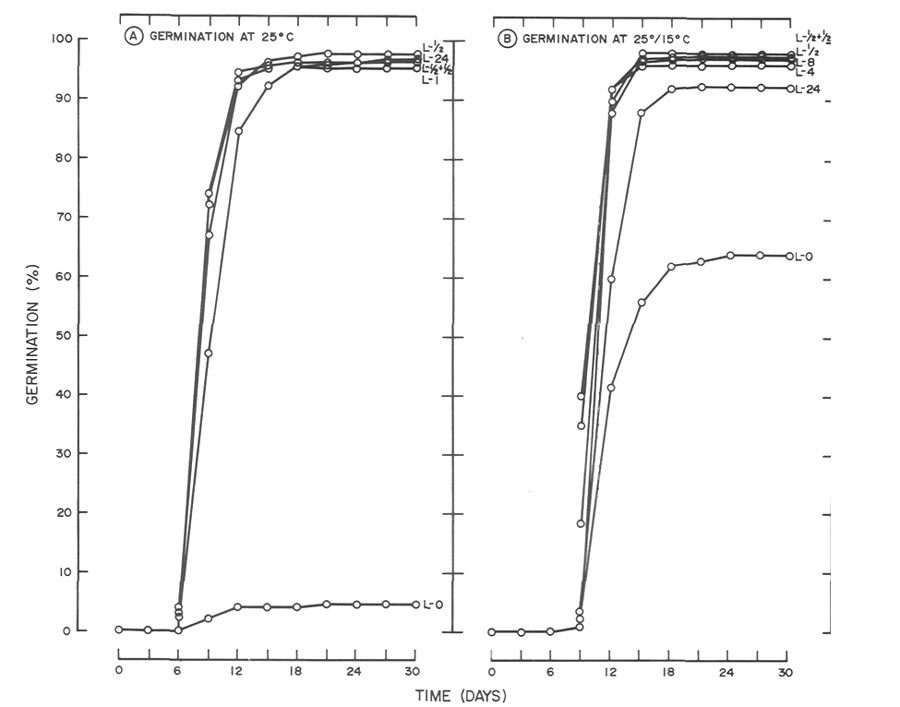
|
|
Fig. 3. Influence of light and temperature on seed germination of
R. catawbiense
.
(A) germinated at 25°C(77°F) with daily photoperiods (L) ranging from total darkness (L-0) to 24 hr. (L-24). Data for photo-periods of 2, 4, 8 and 12 hr. were omitted since germination was similar to the 1/2-hr, light treatment (B) germinated at 25°15°C (77°59°F) utilizing the same photoperiods as in A. Data for photoperiods of 1, 2, and 12 hr. were omitted since germination for the 1 hr. photoperiod was similar to the 8 hr., the 2 hr. was similar to the 4 hr. and the 12 hr. was similar to the 1/2 + 1/2 hr. light treatment. |
Results and Discussion
Regardless of temperature, seeds of
R. maximum
required light for germination (Fig. 2), whereas
R. catawbiense
did not (Fig. 3). At 25°C (77°F), 30-day germination of
R. catawbiense
in darkness was 5%, and with photoperiods > 1/2 hr., germination was > 95% (Fig. 3A). At 25°/15°C (77°/59°F) germination of 64% was achieved in darkness by day 30, which rules out a light requirement at this temperature (Fig. 3B). However, subjecting seeds to daily photoperiods as short as 1/2 hr. increased germination by approximately 30%.
| Table 1. Influence of photoperiod on cumulative seed germination of Rhododendron maximum and Rhododendron catawbiense for days 9 to 30. | |||||||||
|
Temp.
(°C) |
Photo-period z | Time (days) | |||||||
| 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | ||
| R. maximum | |||||||||
| 25° | L | ** | ** | ** | ** | ** | ** | ** | ** |
| Q | NS | ** | ** | ** | ** | ** | ** | ** | |
| 25/15° | L | NS | ** | ** | ** | ** | ** | ** | ** |
| Q | NS | ** | ** | ** | ** | ** | ** | ** | |
| R. catawbiense | |||||||||
| 25° | L | ** | ** | NS | NS | NS | NS | NS | NS |
| Q | ** | ** | * | NS | NS | NS | NS | NS | |
| 25/15° | L | ** | * | NS | NS | NS | NS | NS | NS |
| Q | * | NS | ** | * | * | * | * | * | |
| Z NS, *, ** indicates non-significant, significant (p = 0.05) and highly significant (p = 0.01) linear (L) or quadratic (Q) response, respectively. | |||||||||
Analysis of variance showed that for each species, photoperiods, time and their interaction were highly significant. Thus, regression analysis was conducted on cumulative germination within each temperature for each 3-day interval (Table 1). The regression analysis did not include data for total darkness or the split photoperiod (1/2 + 1/2 hr.).
At 25°C (77°F) a highly significant linear germination response of R. maximum to photoperiod was noted beginning at day 9 and continuing to day 30 (Table 1, Fig. 4A). A similar quadratic effect was also observed except the response began at day 12. No significant response to photoperiod was noted at day 9 for seeds germinated at 25°/15°C (77°/59°F) (Table 1, Fig. 4B). Beginning at day 12 and continuing to day 30, both linear and quadratic responses were highly significant at this temperature.
For seeds of R. catawbiense germinated at 25°C (77°F), highly significant linear and quadratic responses were noted at days 9 and 12 (Table 1, Fig. 5A). By day 15 the linear response was non-significant and continued to day 30. The quadratic influence remained significant (5% level) but by day 18, the relationship became non-significant and remained unchanged to day 30. Significant responses at days 9 and 12 reflected inhibition of germination at the 24-hr, photoperiod (Table 1, Fig. 5A). Inhibition lasted for 9 days and by day 18, germinated for all photoperiod treatments with the exception of total darkness ranged from 95 to 97%.
Inhibition of germination of R. catawbiense by particular photoperiods at 25°/15°C (77°/59°F) was more pronounced since an alternating temperature can partially substitute for a light requirement (Fig. 5B) (10). A highly significant linear response was noted at day 9, decreasing to significant at day 12 and becoming non-significant thereafter (Table 1). The quadratic response was significant at day 9, non-significant at day 12, highly significant at day 15 and significant thereafter (Table 1). At 25°/15 °C (77°/59°F) inhibition was first noted at day 9 for photoperiods > 8 hr. (Fig 5B). By day 15, cumulative germination at all photoperiod treatments with the exception of total darkness and 24-hr, ranged from 96 to 97%. Germination for the 24 hr. photoperiod was 88% which still reflected some inhibition. This increased to 92% by day 18 and essentially remained unchanged to day 30. Although a difference of 5% might seem slight considering the high germination for all photoperiods > 1/2 hr., it appeared inhibition was still evident at day 30 for seeds exposed to constant light (Fig. 5B).
The need for light for seed germination of R. maximum (Fig. 2) agrees with the general statement that seeds of species of Rhododendron require light for germination (1, 2, 7). These findings also agree with those of Romancier (8). At 25°C (77°F), 30 day germination of R. maximum was < 17% for photoperiods < 4 hr. Doubling the photoperiod from 4 to 8 hr. increased germination from 17 to 70%. An additional 4-hr increase to 12 hr. boosted germination to 79%. Finally, a further two-fold increase to 24 hr. did not appreciably influence germination (82% at day 30).
One might view 79 to 82% germination of R. maximum as excellent (Fig. 2A) but germination at 25°/15°C (77°/59°F) was even better. By day 18, 86 to 90% germination was noted for photoperiods > 4 hr. (Fig. 2B). At day 30, the response increased to 95 to 98% and this included the 2 hr. photoperiod. Higher germination at 25°/15°C (77°F/59°F) with relatively short photoperiods, in comparison to reduced germination at 25°C (77°F) with equivalent or longer photoperiods, demonstrates the alternating temperature partially overcame the light requirement when photoperiod was limiting (Fig. 2). A similar response to alternating temperature by seeds of flame azalea was reported by Malek et al. (6).
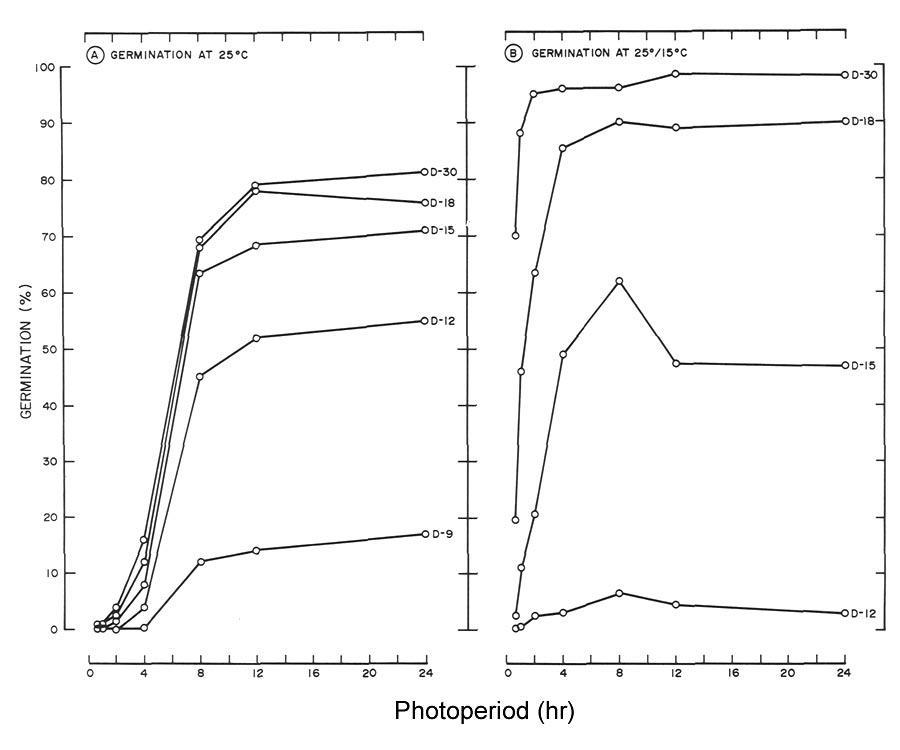
|
|
Fig. 4. Cumulative seed germination of
R. maximum
as influenced by photoperiod at days (D) 9 to 30
(A) germinated at 25°C (77°F). Data for days 21, 24 and 27 were omitted since they were similar to day 30 (B) germination at 25°/15°C (77°/59°F). Data for days 9, 21, 24 and 27 were omitted since germination at day 9 was similar to day 12, and days 21, 24 and 27 were similar to day 30. |
|
|
Fig. 5. Cumulative seed germination of
R. catawbiense
was influenced by photoperiod at days (D) 9 to 30.
(A) germinated at 25°C (77°F). Data for days 18, 21, 24 and 27 were omitted since they were similar to day 30. (B) germinated at 25°/15°C (77°/59°F). Data for days 18, 21, 24 and 27 were omitted since germination was similar to day 30. |
Poor germination of R. catawbiense (5% at 30 days) at 25°C (77°F) in total darkness was overcome by daily photoperiods as short as 1/2 hr. (Fig. 3A). By day 15, 92 to 97% germination was noted for all photoperiods > 1/2 hr. Interestingly, on day 9, 67 to 75% germination was observed for photoperiods of 1/2 to 12 hr. while germination under constant light was 47%. As mentioned previously, germination was inhibited by the longer photoperiods. However, by day 18, germination of all photo-period treatments > 1/2 hr. ranged from 95 to 98%. Similar inhibition was also observed at 25°/15°C (77°/59°F) (Fig. 3B). On day 9, 41% germination was noted for the 4 hr. photoperiod. At the same time, germination of 19,8 and 2% was observed for seeds receiving photo-periods of 8, 12 and 24 hr., respectively. By day 15, germination of all seeds exposed to photoperiods of 1/2 to 12-hr, ranged from 96 to 98% while seeds under constant light were still inhibited (88%). By day 18,germination under all photoperiods except darkness was 92 to 98%.
Relatively high germination (64%) of R. catawbiense in darkness at 25°C/15°C (77°/59°F) was surprising in comparison to low germination (5%) also in darkness at 25°C (77°F) (Fig. 3). Greater germination at the alternating temperature in comparison to the constant temperature would not be considered unusual, but the magnitude of the response led the authors to repeat the study, utilizing seeds from the same lot. Results were similar leading to speculation whether the responses in darkness at 25°C (77°F) and 25°/15°C (77°/59°F) were characteristics of the seed lot or the provenance. To answer the former, germination of seeds in total darkness at 25°C (77°F) and 25°/15°C (77°/59°F) was compared using seeds from two different lots. One lot, whose germination response is presented in Figs. 3 and 5, consisted of seeds collected in fall 1986, and the second consisted of seeds collected from the same population in fall 1988 and stored under identical conditions as the 1986 seeds. Results (data not presented) showed that regardless of temperature, dark germination of the 1988 seeds was negligible < 1%). Further work showed that germination in light for the 1988 seeds was similar to that in Figs. 3 and 5. Since collection dates varied for the two seed lots, this raises the question whether the response in darkness was influenced by the environmental conditions under which the seeds developed. Another possibility might involve loss of light sensitivity as a result of storage conditions or simply loss of light sensitivity with time (aging). Both the aforementioned are deserving of further study.
Seed germination > 90% of R. catawbiense can be achieved at temperatures of 25°C (77°F) and 25°/15°C (77°F/59°F) with daily photoperiods as short as 1/2 hr. Similar results can be attained at 25°F/15°C (77°/59°F) for R. maximum with a 2-hr, photoperiod. Although short photoperiods stimulate high germination, subsequent growth and development of seedlings would undoubtedly benefit from much longer photoperiods.
Literature Cited
1. Cho, M.S., J.H. Jung and D.Y. Yearn. 1981. Studies on seed germination of rhododendron plants. J. Korean Soc. Hort. Sci. 22:107-120.
2. Dirr, M.A. and C.W. Heuser, Jr. 1987. The Reference Manual of Woody Plant Propagation: From Seed to Tissue Culture. Varsity Press, Athens, GA.
3. Downs, R.J. and J.F. Thomas. 1983. Phytotron procedural manual for controlled environment research at the Southeastern Plant Environment Laboratory. N.C. Agr. Res. Serv. Tech. Bui. 244 (revised).
4. Leach, D.G. 1961. Rhododendrons of the World. Charles Scribner's Sons, New York.
5. Liberty Hyde Bailey Hortorium. 1976. Hortus Third: A Concise Dictionary of Plants Cultivated in the United States and Canada. 3rd ed. Macmillan Publishing Co., New York.
6. Malek, A.A., F.A. Blazich, S.L. Warren and J.E. Shelton. 1989. Influence of light and temperature on seed germination of flame azalea. J. Environ. Hort. 7:109-111.
7. Olson, D.F., Jr. 1974. Rhododendron L. rhododendron, p. 709-712. In: C.S. Schopmeyer (Tech. Coordinator). Seeds of Woody Plants in the United States. Agr. Hdbk. 450. U.S. Dept. Agr., Forest Serv., Washington, D.C.
8. Romancier, R.M. 1970. Ecology of the seedling establishment of Rhododendron maximum L. in the Southern Appalachians. Ph.D. Thesis, Duke Univ., Durham, N.C.
9. SAS Institute. 1985. SAS User's Guide: Statistics, Version 5 Edition. SAS Institute, Cary, N.C.
10. Toole, E.H., V.K. Toole, H.A. Borthwick and S.B. Hendricks. 1955. Interaction of temperature and light in germination of seeds. Plant Physiol. 30:473-478.
