JARS v47n4 - Natural History and Recommendations for Control of the Rhododendron Stem Borer, Oberea myops
Natural History and Recommendations for Control of the Rhododendron Stem Borer, Oberea myops
Joseph D. Culin, Clyde S. Gorsuch, Theresa M. Pizzuto
Department of Entomology, Clemson University
Abstract
The biology of the rhododendron stem borer,
Oberea myops
Haldeman, was examined in rhododendrons and deciduous azaleas. This long horned beetle, which is also referred to as the azalea or blueberry stem borer, occurs in the eastern United States from Maine to northern Georgia and west into eastern Tennessee. Adult activity and oviposition occur in the western Carolinas from late May through mid-July. Following egg hatch, larvae tunnel through the center of stems toward the ground surface. This activity may result in severe weakening of the stem leading to breakage during heavy wind, rain or snow. In rhododendrons, the majority of the individuals completed development in a single year, although some required two years. In deciduous azaleas, most individuals completed development in two years while a few required three years. In deciduous azaleas no individuals completed development in their first year.
Mechanical control methods for this potentially damaging pest are discussed.
Host range.
The rhododendron stem borer,
Oberea myops
Haldeman, is a longhorned beetle (Coleoptera: Cerambycidae) which can develop on a wide range of ericaceous host plants that includes both cultivated and native rhododendrons (
Rhododendron
), mountain laurel (
Kalmia
), evergreen and deciduous azaleas (
Rhododendron
),
Pieris japonica
,
Oxydendrum arboreum
and blueberries (
Vaccinium
) (Driggers 1929, Beckwith 1934, White & Hamilton 1935, Lehman 1984, Galle 1987, Johnson & Lyon 1988, Culin & Gorsuch unpublished data).
Geographic distribution.
Johnson & Lyon (1988) report that
O. myops
is a native insect likely to be found throughout the range of its ericaceous hosts. In an attempt to determine the distribution of this borer, we contacted curators of all major insect collections east of the Mississippi River, and any private collectors who dealt primarily with long-horned beetles. Based on the records of
O. myops
in these collections, and published reports of this beetle from Pennsylvania (Lehman 1984, Kirk & Knull 1926, Champlain et al. 1925), Ohio (Knull 1946), and New Jersey (Driggers 1929, Beckwith 1934, White & Hamilton 1935), a distribution map was prepared (Fig. 1). It appears that the primary distribution of this beetle is along the Appalachian Mountains from Maine to western South Carolina and northern Georgia. Although there are several records of coastal populations in North Carolina and Virginia we believe that beetles were introduced into these areas rather than occurring there naturally.
Information presented here is based on the biology of O. myops in cultivated 'English Roseum' rhododendrons at Clemson University (Clemson, Pickens Co., South Carolina), and in a mixed variety planting of deciduous azaleas at Biltmore Estates (Asheville, Buncombe Co., North Carolina). Additional information on adult activity was gathered at Mountain Creek Rhododendrons (Marietta, Greenville Co., South Carolina) and at Hurricane Gap Nursery (Zirconia, Henderson Co., North Carolina).
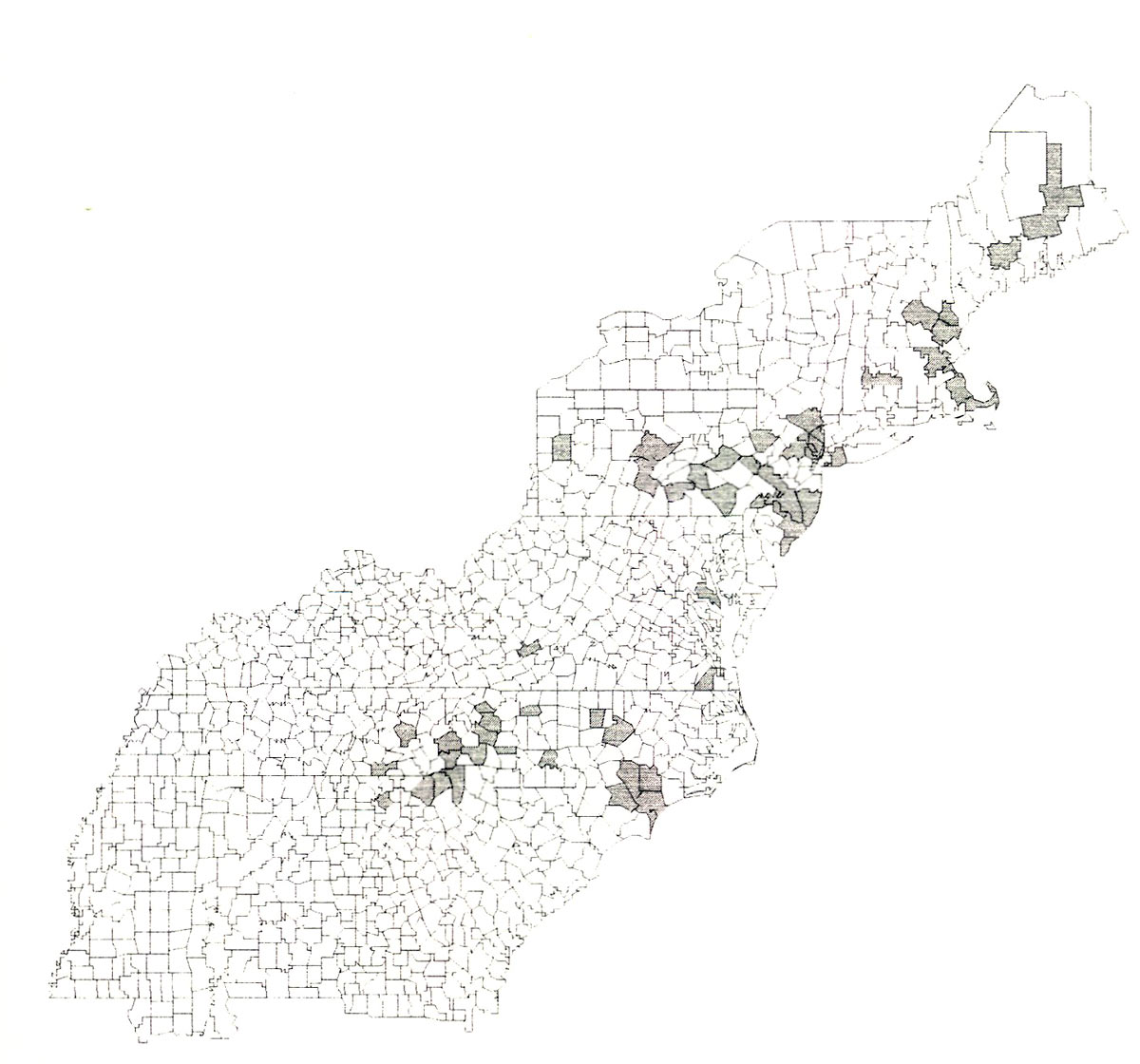
|
| Figure 1. Distribution map of Oberea myops . |
Descriptions of Life Stages and Damage Adults.
Adults are slender beetles averaging 16.2 mm (⅝ in) in length and are active from late May through mid-July. The body is copper colored with three brown stripes running the length of the wing covers. There are two black spots on the "shoulders" of the wing covers and two more on the rounded plate behind the head. The head has two kidney bean shaped black eyes at the base of elongate black antennae (Fig. 2). Adults feed primarily on the underside of leaf midribs and to a much lesser extent on the outer tissue layer of new growth stems. Although feeding results in leaf curling or wilting, this damage is primarily aesthetic.

|
| Figure 2. Adult Oberea myops . |
Prior to oviposition, a female chews two girdles around new growth stems (Fig. 3). Distance between girdles ranged from 8 to 26 mm (5/16 to 1 1/16 in). The female then either makes a longitudinal slit in the bark between the two girdles and places a single egg under the bark, or inserts the egg through an area of the girdle without splitting the bark. Eggs average 4 mm (5/32 in) in length (Fig. 4) and require approximately 10 days to hatch under natural conditions. Oviposition activity generally does not result in the immediate wilting of rhododendrons, although it does cause rapid wilting (referred to as "flagging") in girdled stems of hosts such as deciduous azaleas and blueberries.

|
| Figure 3. Oviposition wounds (girdles) on new growth. |
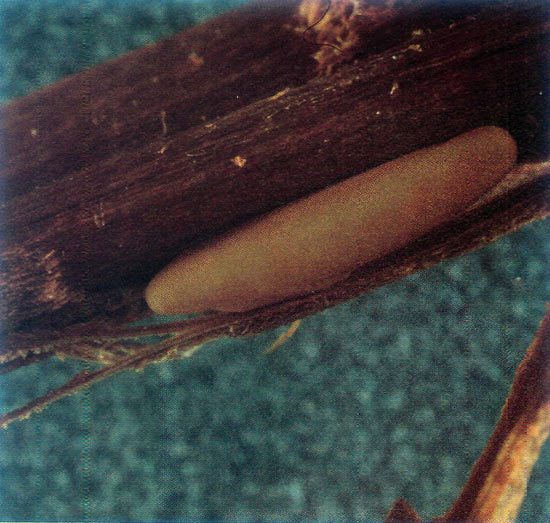
|
|
Figure 4.
Oberea myops
egg.
Bark has been peeled back to expose the egg. |
Larvae.
Larvae are commonly referred to as borers (Fig. 5). After hatching, they bore upward toward the stem tip for a brief period and then turn and move down into healthy wood below the lower girdle. It is generally not until larval feeding begins that wilting is observed in rhododendrons. Infested stem tips generally break from the plant at the lower girdle shortly after larvae move into healthy wood.

|
|
Figure 5.
Oberea myops
larva.
Stem has been split open to expose the larva. |

|
| Figure 6. Frass expelled from holes in the stem. |
As larvae bore down through the stem, they regularly open small (1 mm; 1/32 in) holes in the stem from which sawdust-like waste, called frass, is expelled. The average distance between these holes is similar in both rhododendrons and deciduous azaleas, and averages 6.2 cm (2½ in) (Table 1). As frass is expelled from these holes, it accumulates in leaf axils, on leaf surfaces and on the soil surface and can be used to locate active borers (Fig. 6).
| Table 1. Average distance in cm [inches] between frass clean-out holes. | ||||
| Host Plant |
year of egg
deposition |
developmental year | ||
| 1st | 2nd | 3rd | ||
| Rhododendron | 1989 | 7.3 [2.9] | 4.3 [1.7] | -- |
| Deciduous Azalea | 1989 | no data | 8.6 [3.4] | 6.1 [2.4] |
| 1990 | 5.1 [2.0] | 7.9 [3.1] | no data | |
| 1991 | 4.4 [1.7] | no data | no data | |
We have found that the life cycle varies between the two types of host plants examined. In rhododendrons, larvae require either one or two seasons to complete their development. Of the 28 larvae we followed, 25 completed development during the first year and 3 during the second. During their first season, larvae tunneled an average of 56.6 cm (22¼ in) and opened an average of 7.8 frass clean-out holes. Those requiring a second season to complete development moved an average of 13 cm (5⅛ in) and opened an average of 3.0 holes (Tables 2 & 3).
| Table 2. Average total distance in cm [inches] tunneled in stems. | ||||
| Host Plant |
year of egg
deposition |
developmental year | ||
| 1st | 2nd | 3rd | ||
| Rhododendron | 1989 | 56.6 [22.3] | 13.0 [5.1] | -- |
| Deciduous Azalea | 1989 | no data | 70.5 [27.8] | 14.9 [5.9] |
| 1990 | 21.8 [8.6] | 51.8 [20.4] | no data | |
| 1991 | 12.9 [5.1] | no data | no data | |
| Table 3. Average number of frass clean-out holes opened. | ||||
| Host Plant |
year of egg
deposition |
developmental year | ||
| 1st | 2nd | 3rd | ||
| Rhododendron | 1989 | 7.8 | 3.0 | -- |
| Deciduous Azalea | 1989 | no data | 8.5 | 2.8 |
| 1990 | 3.3 | 6.1 | no data | |
| 1991 | 2.6 | no data | no data | |
In deciduous azaleas, larvae require either two or three seasons to complete development. Of the 62 larvae we have monitored in azaleas, the majority completed development during the second season while a few required a third season. Larval activity reflects this difference with larvae tunneling relatively short distances and opening few holes during their first season (17.35 cm; 3 holes), followed by considerable activity during their second season (61.2 cm; 7 holes). Those requiring a third season only tunnel a short distance and open a few holes (14.9 cm; 3 holes) (Tables 2 & 3).
When a larvae has completed its development it enters the pupal stage where it transforms to an adult. This stage appears to occur within the stem which has been plugged with frass (Lehman 1984, Beckwith 1934, Driggers 1929). In small plants larvae often reach the crown where they may hollow out a large area in which they pupate. This type of crown damage often kills small plants. In larger plants, larvae complete development before reaching the crown, and appear to hollow out an area within the stem. After they become an adult, they open an elongate exit hole in the stem and leave the plant. This exit-hole damage may weaken a stem to the point where it will break.
In both types of host plants, larvae remaining in the plants become inactive over an extended period during the fall, with no signs of activity by early December. In addition to their tunneling activity, larvae that remain in the plants during the winter often cause another type of damage prior to becoming inactive in the fall. In both types of host plants, major branches were pruned out by the larvae. This occurs as larvae inside the stems girdled outward from the tunnel to the bark. Although this does not result in immediate damage, these branches break from the plants under heavy rain, snow or windy conditions. If these larvae have moved far down a major branch, this activity can result in loss of form as a considerable portion of a plant may break out.
Control strategies.
At this time, the most efficient control strategies for this pest are cultural techniques aimed at larval control. In mid- to late-summer as larvae begin to feed in new growth, stem wilting and frass are usually obvious. Infested stems should be pruned from the plant at this time. The stem should be cut below the lowest frass clean-out hole. Larvae can not disperse from pruned stems to healthy plants and so pruned stems can simply be dropped in the bed if desired (Driggers 1929, Beckwith 1934). However, Lehman (1984) suggests removing and burning infested material. After pruning, the stem below the cut should be examined to ensure that the borer remains in the portion that was removed. If a hole remains in the center of the stem the borer has already migrated below the cut and further pruning may be necessary to remove it.
Two alternative approaches, which may be effective if the borer remains in the plant below the cut, are also useful if a borer is discovered in an older part of the plant that would not be amenable to pruning. In this case the borer can be killed either by slowly inserting a thin, flexible wire into the tunnel until the bottom is reached, or some lightweight oil can be placed into the tunnel which will suffocate the borer. The wire technique was utilized extensively in blueberries in New Jersey in the 1920s (Driggers 1929) and is recommended by Galle (1987) for use in azaleas. Phil Marucci (personal communication) has reported that growers used the oil technique in blueberries in New Jersey in the early- to mid-1900s. Both the wire and oil techniques require a cut stem in order to be effective. Frass clean-out holes opened by the borer can not be used for insertion of the wire or oil as they are typically oriented at an upward angle. Inserting a wire into one of these holes would result in a wire going up toward the stem tip rather than down the tunnel to where the borer is located.

|
Acknowledgements
We would like to thank the American Rhododendron Society for providing the funding to initiate this project and construct the screen-house facility. We also would like to thank Biltmore Estates (Asheville, NC), Nick & Neala Anastos (Mountain Creek Rhododendrons) and David Dethero (Hurricane Gap Nursery) for allowing us to work on their properties. Ted Richardson (The Rhododendron Farm, Mountain Home, NC) donated the rhododendrons used in this study. Jimmy Pilgrim, Darrel Walker and Lori Brown assisted in taking samples. This is Technical Contribution No. 3158 of the South Carolina Agricultural Experiment Station, Clemson University.
References
Beckwith, C. S. Blueberry stem borer. New Jersey Agricultural Experiment Station Circular 320. New Brunswick, NJ; 1934.
Champlain, A. B.; Kirk, H. B.; Knull, J. N. Notes on Cerambycidae (Coleoptera). Entomol. News. 36: 139-142; 1925.
Driggers, B. F. Notes on the life history and habits of the blueberry stem borer, Oberea myops Hald., on cultivated blueberries. J. New York Entomol. Soc.37:67-73; 1929.
Galle, F. C. Azaleas. Timber Press, Portland, OR. 519 pp.; 1987.
Johnson, W. T.; Lyon, H. H. Insects that feed on trees and shrubs. 2nd ed. Cornell Univ. Press, Ithaca, NY. 556 pp.; 1988.
Kirk, H. B.; Knull, J. N. Annotated list of the Cerambycidae of Pennsylvania (Coleoptera). Can. Entomol. 58: 39-36; 1926.
Knull, J. N. The long-homed beetles of Ohio (Coleoptera: Cerambycidae). Ohio Biol. Surv. Bull. 39. (Vol. 7, No. 4, pp. 133-343). The Ohio State Univ., Columbus, OH; 1946.
Lehman, R. D. Azalea stem borer, Oberea myops Haldeman. Pennsylvania Dept. of Agric. Bureau Plant Industry. Entomol. Circ. 86; 1984.
White, R. P.; Hamilton, C. C.; Diseases and insect pests of rhododendron and azalea. New Jersey Agricultural Experiment Station Circular 350. New Brunswick, NJ; 1935.
