JARS v48n3 - An Improved Chromosome Staining Method Applied to the Study of Colchicine Effects in Rhododendron
An Improved Chromosome Staining Method Applied to the Study of Colchicine Effects in Rhododendron
John E. Eiselein
El Cerrito, California
Summary
An orcein based staining procedure is presented that has proven successful in chromosome analysis of rhododendron root tip in seedlings derived from six different colchicine treated plants. While somatic effects expressed in the parent plants were characteristic of colchicine treatment and presumed a manifestation of polyploidy, the generative cells were not affected - the seed remained diploid. Exposure of bud or explant tissue is discussed as more probable candidates for successful nuclear conversion.
Colchicine, a potent mitotic inhibitor, can induce chromosome doubling in dividing plant cells. This property of genetic potentiation, discovered in 1937, has been intensively exploited to create many improved plant varieties. A detailed review of this pioneer work can be found in the volume Colchicine in Agriculture, Medicine, Biology, and Chemistry by O. J. Eigsti and Pierre Dustin Jr., 1955, published by Iowa State University Press.
When rhododendron seedlings are subjected to colchicine, certain characteristic changes have been reported as developing in some fraction of these plants as they mature. Effects include ornamental enhancing features involving leaf and flower form. It is generally assumed these phenotypic changes are an expression of induced polyploidy (2), (3). Nuclear analysis, confirming chromosomal changes by actual count, has, however, infrequently been performed, owing, in part, to the lack of suitable staining techniques capable of consistently differentiating the very tiny, stain refractory, rhododendron chromosomes.
Acetocarmine, the stain of choice for plant chromosomes, has proved wanting when used for rhododendron work. Repeatedly, staining results have been poor, reported as "minimal" (3), "unsatisfactory" (4), and "non specific" (5). When my own efforts to stain rhododendron chromosomes with acetocarmine failed I sought an alternative, and found a 1941 study by L. LaCour reporting that orcein, a stain seldom used in plant work, had proved superior in staining chromosomes, especially the tiny varieties. Using an acetic acid fix with this stain, LaCour claimed the combination was more easily regulated, preserved preparations better and, most importantly, was much more selective in distinguishing chromatic from cytoplasmic background than acetocarmine (5).
While LaCour's presentation was rather general, assuming, I suppose, a technically experienced audience, I was, nevertheless, as a novice plant worker able, with some experimentation, to work out procedural details that I found satisfactory for demonstrating rhododendron chromosomes in meristem root cells. I am presenting this procedure here, hoping the details will prove of interest to others who might wish to venture into this discipline. I follow my staining exposition with a small study wherein I apply the orcein procedure to nuclear analysis of seedlings produced by colchicine treated rhododendrons that exhibit the classical somatic characteristics associated with polyploidy induction.
Acetic-Orcein Staining Technique
Slide preparation for micro study of chromosomes is a three stage process: fix, stain, mount. These steps, while easy in principle, are difficult in practice, owing to the fragility of the tiny meristematic cell cluster that must be guarded against mechanical disruption as the seedling is maneuvered through the various solutions and finally brought under the knife and cover slip.
I cannot overemphasize that physical considerations are as critical in the process as reagents if the metaphase nuclei are to be preserved and suitably stained for analysis.
Chemicals and Equipment Required
1. Orcein stain. I used an available Orcein Natural Stain, produced by Spectrum Mfg. Corp., 1422 San Pedro Street, Gardena, CA 90248.
2. Visible-light microscope, equipped with 100X, 1.3 N.A., oil immersion objective and an oil-immersion, rheostatic controlled sub-stage condenser.
3. A couple of improvised sub-stage illumination lights of the sort featured in Figures 2 and 3, consisting of a 50-watt spot and a sub-slide fluorescent lighted table-slide-mount.
4. Glacial acetic acid, reagent grade, methanol, reagent grade, distilled water.
5. Pre-cleaned (write on) No. 1 microscopic glass slides, 1 to 1.2mm thick.
6. No. 4 Bard Parker knife handle fitted with No. 20 blades.
7. Variety of Pyrex beakers.
8. 1 and 5ml syringes.
9. Needle-nosed forceps.
10. Ring stand and clamps.
11. Alcohol burner.
12. Clear finger nail lacquer.
13. Amber colored glass storage bottles.
14. Graduated glass cylinders, 10 and 100ml volume.
15. Several small Petri dishes.
16. Fine porosity quantitative grade filter paper.
17. Absorbent blotting tissue.
18. Watch glass dishes.
19. Cover slips, No. 2 (plastic or glass, as optic specification require).
20. Finger cot (to protect thumb from stain).
Reagents
1. 1% orcein in 45% acetic acid. Standard working stain for soft, primary root study (three-month shelf life only).
2. 1% orcein in 45% acetic acid plus 0.1N HCL for staining hardened secondary root tissues.
3. Fixative. 30% acetic acid in methanol.
4. Glacial acetic acid, post fix rinse.
5. 2.2% orcein, made up in pure glacial acetic acid for long storage - will develop scum that must be filtered off. Dilute to 45% acetic acid when required for use (6).
Reagent Preparation
Standard working stain:
Prepare 1% orcein by dissolving 1 gram of orcein powder in 45ml of near boiling glacial acetic acid, when cool add 55ml of distilled water, stir (glass rod), filter through qualitative filter, store in an amber bottle with plastic seal in the cap (avoid metal sealer).
HCL acidified stain: Make up 1N HCL by adding 3.6ml of the acid to 100ml of distilled water. Add 1ml of this to 10ml of the standard working stain.
Fixative: To make up 30% acetic acid fixative add 30ml of glacial acetic acid to 70ml of reagent grade methanol. Acetic acid, post fix solution. Use pure glacial acetic acid.
Staining and Slide Preparation
Fixation:
Add no more than six seedlings to a small Petri dish (5cm in diameter) containing 10 to 15ml of 30% acetic acid fixative solution, cover, maintain temperature between 75°F and 85°F for 20 minutes while occasionally agitating with a gentle circular motion. Transfer fixed seedlings through post-fix glacial acetic acid for two-to-three minute exposure.
Stain: Place the fixed seedlings into a small Petri dish containing 10 to 15ml of the orcein standard stain for primary root tip staining, or, for secondary root tips, use 0.1N HCL acidified orcein stain, cover, incubate at 75°F and 85°F for one hour, agitate occasionally by gentle rotation.
Heating: Place stained seedlings into 8ml of (standard or HCL) orcein stain in a watch glass and gently heat for two to three minutes over an alcohol flame. Do not boil.
Smash and Mount: Illuminate watch glass from below using spot light fixture. Clamp a glass microscopic slide onto fluorescent sub-lighted mount, add a small drop of fresh stain to center of the slide, transfer one seedling into the stain, cut no more than 1 or 2mm of the radicle tip, discard epicotyl, police the fly-speck-sized meristem tissue to stain-drop center, overlay with cover slip. Remove slide to a firm, smooth table top. While keeping the cover slip from moving, apply thumb pressure very, very slowly until maximum pressure is achieved. Hold pressure steady for 20 seconds, then release very slowly. Blot slide dry with tissue, then gently heat back of slide over an alcohol flame until comfortably warm to touch, then ring with a band of lacquer and allow to dry before microscope study.
|
|
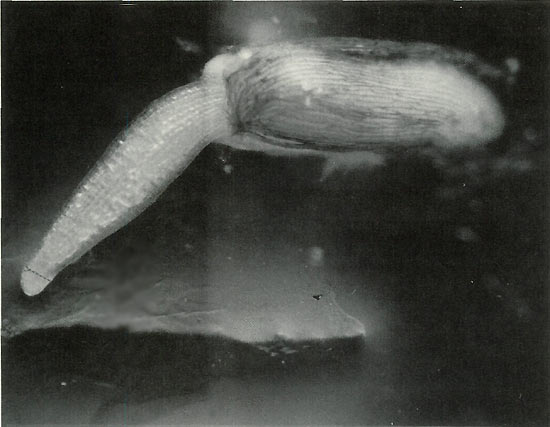
Figure 1. 50X Magnification. Germinating seed is shown at the ideal
stage for staining, when the meristem is still compressible. As it grows, it will thin and harden, and there will be a lower concentration of mitotic spreads suitable for analysis. Arrow indicates approximate point where meristem should be incised after staining. Only a speck of tissue is needed; if more than a couple millimeters long it will not flatten under the cover slip and air bubbles will develop and spoil the preparation. HCL added to the stain softens tissue, but differential staining of the chromatin is reduced. Secondary root tip presents this problem. Satisfactory counting can be achieved, but requires greater effort. Photo by John E. Eiselein |
Important Considerations
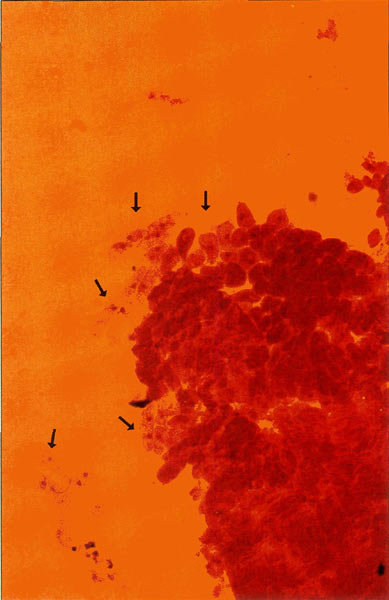
Incubate seed on fine porosity qualitative filter paper. Avoid paper towels, especially for secondary root study. Radicle tips will penetrate large porosities and be lost. Stain at first sign of germination when doing primary root study, before hypocotyl exceeds seed coat in length as shown in Figure 1. Root tip is soft and compressible at this time and mitotic cells of interest present themselves in a loose array, floating in a fluid-like matrix (permitting mobility?) surrounding the dense meristem apex. These are the cells that must be preserved. See colored photo Figure 4.
At all times, when handling seedlings, seize them by the seed coat, avoid touching them on the tip. For controlled handling, illumination of the dark staining solution is required. Lighting aids as shown in Figures 2 and 3 will prove of great help.
|
|
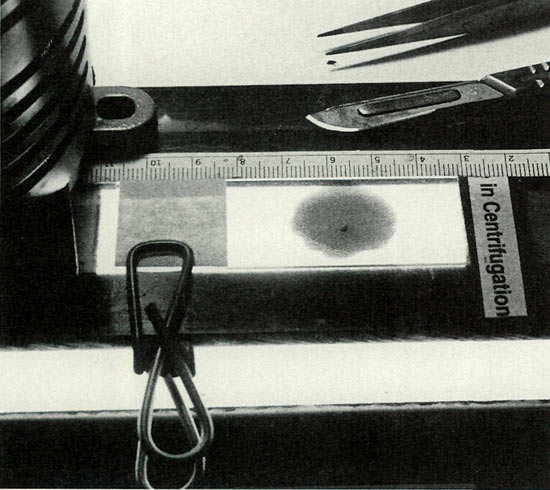
Figure 2. A masked slide mount, providing a clamp, guide stops
and sub-illumination, was improvised from an under-the-counter fluorescent fixture and found indispensable for the required precision cutting of the meristem. Photo by John E. Eiselein |
Note on cutting: The knife blade must be kept ultra sharp to separate the tiny bit of meristem tissue without macerating it. Acetic acid quickly dulls the blade. Its useful edge can be preserved longer if, after each cut, the blade is rinsed in distilled water, but it must be carefully dried before reuse as water will dilute the stain. Often, upon cutting, the section will cling to the blade. It is helpful to have a syringe loaded with stain parked nearby to rinse the sectioned piece back onto the slide.
|
|
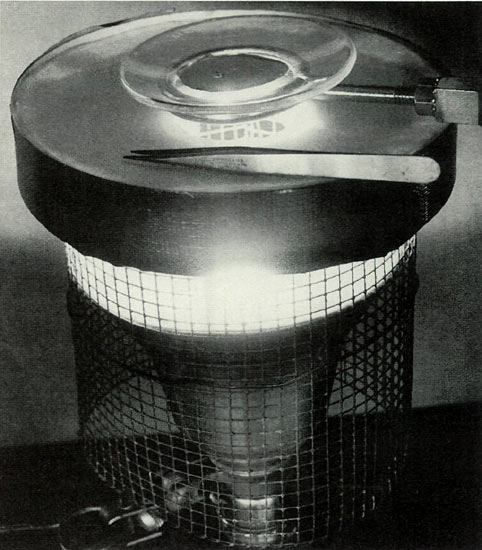
Figure 3. Successful manipulation of seed in darkened
orcein stain requires intense illumination. A simple, well ventilated 50-watt spot, masked with a perforated shield produced satisfactory working light. Photo by John E. Eiselein |
A Chromosome Study Of Colchicine Effects In
Rhododendron
Introduction
How predictive of generative polyploidy are the gross somatic changes developing over time in plants with a history of colchicine treatment as seedlings? Do the assumed somatic nuclear changes include the sex cells?
David E. Wagner of Burtonsville, Md., an experienced hybridist, practiced in the use of colchicine, has helped with this question. He provided me with seed harvested from six different colchicine induced Rhododendron fortunei plants that had been aggressively treated with a 2% aqueous solution for four days in an aerated suspension when they were germinating seedlings. As mature plants they were fertilized with pollen obtained from diploid R. 'Janet Blair'. The R. fortunei were presumed tetraploid induced on the basis of significant somatic changes characteristically associated with colchicine treatment. These include large, disproportionately wide, heavy textured leaves, enlarged, heavy textured flowers, displaying multiple style and enlarged stigma. Anthers were absent.
Negative Results
In spite of the abnormal secondary sexual anatomy manifest in the six
R. fortunei
plants, the diploid pollen from R. 'Janet Blair' produced fertile seed in all of them. This was germinated and the primary root tissue was stained with acetic-orcein for chromosome analysis.
It would be expected, if the colchicine treated R. fortunei were tetraploid, then crossed with diploid pollen, the resulting seed should exhibit triploid nuclei. This, surprisingly, however, was not the case. When the chromosomes of the nuclei were counted all six of the putative triploids displayed diploid chromosome complements.
The colchicine effect, however strongly expressed somatically, did not induce polyploidy in the gametes. The absence of polyploidy in the female generative cells seems surprising, considering the abnormalities manifest in style and stigma.
Consistent with these diploid findings was Wagner's observation that when seed from the colchicine plants had been sown in previous years, the second generation bore normal leaf and flower. Clones made from the colchicine plants, however, reportedly did maintain their somatic divergence from the plant form peculiar to the species R. fortunei , assuming, therefore, an interpretation of genetic rather than temporary physiological effects. Regrettably, I did not have access to the parent plants growing out in Maryland to confirm their polyploidy by chromosome analysis.
|
|
Figure 4. 200X photomicrograph of an orcein
stained meristem. Islands of cells presenting the metaphase nuclei that we require for chromosome analysis drift loosely around the apical mass and are easily displaced, emphasizing the need for careful handling. Photo by John E. Eiselein |
Discussion
While we should not generalize from a single study, these results are consistent with the idea that colchicine induction of sexual ploidy might be a low probability event in woody plants of high order such as
Rhododendron
. It should be emphasized that colchicine effects defy easy analysis, since interpretation is complicated by the fact that only that fraction of the cell population in mitotic metaphase at the time of treatment are susceptible to chromosome doubling. Treated plants may sometimes be a mosaic patchwork of variable ploidy, more aptly described as "colchichimeras" rather than "tetraploids." I would like to refer the reader to the interesting and very complex phenomenon of chimerism, as discussed by Richard Tilney-Basset in his fairly recent volume, that bears very importantly on this question (8).
A further complication in interpretation of morphological effects is the fact that colchicine is not only a mitotic inhibitor, but has other significant physiological effects, most notably induction of hormonal imbalances affecting growth rates and differentiation that could impact somatic characteristics (7).
Hybridists seeking polyploidy seed in breeding experiments using colchicine induced parent plants should therefore be cautioned in their reliance on gross morphological changes to indicate tetraploid gametes.
This is not to demean the usefulness of colchicine for horticultural improvement in Rhododendron , but it should be kept in mind that somatic enhancement might be restricted to preservation and reproduction by vegetative cloning, i.e., cuttings, except, of course, in the case where naturally occurring polyploidy rhododendron species, or hybrids derived from them, are used in breeding experiments.
Perhaps, colchicine treatment of flower bud, or tissue culture, will better target gametes for nuclear conversion than treatment of seed. In vitro exposure to colchicine of rhododendron explants has been reported by Paden, Meyers and Rayburn of the University of Illinois, whereby tetraploid conversion was indicated on the basis of increases in chloroplast bodies in epidermal guard cells. A correlation with ploidy was established in sugar beet and maize and indicated in Rhododendron by August Kehr's early observation that epidermal guard cells were larger in tetraploid species than in diploid species (9), (10).
Paden, Meyers and Rayburn reported increased chloroplast counts in guard cells from four of twelve hybrids I had treated with colchicine as seedlings. These results correlate with my own, yet unpublished observation, of tetraploidy in the root tip of all four of these plants as determined by direct orcein stained chromosome study. These specimens are alive and growing but have not bloomed to allow chromosome determination in their gametes. (I believe generative conversion is unlikely.)
I have learned, in a private communication with Dr. Paden, that seed derived from his colchicine treated explants failed to germinate on his first try so that tetraploidy in the gametes has not yet been confirmed, but his in vitro work is going forward and has exciting promise.
Acknowledgments
I would like to express appreciation to August Kehr for his helpful guidance, to David Wagner for providing unusual seed and information necessary for this study, and Don Paden and Lane Rayburn for their epifluorescent analysis of chloroplasts in my leaf material.
References
1. Kehr, A. E. Questions and answers. ARS Bulletin 27:20-23; 1973.
2. Leach, D. G. Rhododendrons of the world. New York: Charles Scribner's Sons; 1961.
3. Tolstead, W. L.; Glenco, J. F. Winter-hardy tetraploids of Rhododendron carolinianum and Rhododendron racemosum , and their tetraploid hybrids. J. Amer. Rhod. Soc. 45: 83-84; 1991.
4. Hosada, T. A., et al. Genetica 26: 407-409; 1953.
5. LaCour, L. Acetic-orcein: a new stain fixative for chromosomes. Stain Technology. 16: 169-172; 1941.
6. Darlington, C. D.; LaCour, L. Stain Technology. 142; 1969.
7. Eigsti, O. J.; Dustin, P. Colchicine. Ames: Iowa State College Press; 1955.
8. Tilney-Basset, R. A. E. Plant chimeras. London: Edward Arnold; 1986.
9. Paden, D. W.; Meyers, M.M. Jr.; Rayburn, A. L. Doubling chromosomes with colchicine treatment in vitro as determined by chloroplast number in epidermal guard cells. J. Amer. Rhod. Soc.44: 162-167;1990.
10. Kehr, A. E. A tetraploid Rhododendron carolinianum . ARS Bulletin. 25:4-7; 1971.
John E. Eiselein is a biomedical research scientist at the University of California, Lawrence Livermore Laboratory, where he has been involved in cancer studies for the past 30 years. His academic training was in marine biology and immunology. He describes himself as "eminently qualified as inexperienced in botanical matters." (His foundation in botanical knowledge is limited to the ARS Journal and two textbooks which he mentions in the article.)
The staining method described is "cookbook simple" and requires only practical knowledge and dexterity and a microscope, John Eiselein assures readers. He hopes to encourage hybridists to take up the microscopy craft if they are doing colchicine work.
