JARS v52n4 - Microprojectile-mediated Genetic Transformation of Rhododendron Hybrids
Microprojectile-mediated Genetic
Transformation of Rhododendron Hybrids
Chi-Ni Hsia and Schuyler S. Korban
Perennial
Plant Biogenetics Laboratory
University of Illinois, Urbana, Illinois
Synopsis
This paper is a report on finding or adapting a feasible method of gene transfer for rhododendrons using helium pressure bombardment devices for introducing selected DNA into two cultivars of evergreen azaleas ('Hino-crimson' and 'Fuchsia'). Marker genes were injected by this method to evaluate successful introduction of the selected DNA into in vitro azalea tissues, and the method proved to be successful.

|
Rhododendrons have complex genetic systems because of their high levels of heterozygosity and existence of breeding barriers (before and after fertilization) among species leading to cross-incompatibility (Williams etal., 1990). Reproductive barriers have been especially reported between the two major subgroups, subgenus Rhododendron (lepidotes) and the other seven sub-genera (elepidotes) (Williams etal., 1990). Although sexual hybridization and selection have been used by plant breeders in traditional breeding programs for many years, the available gene pool is restricted by sexual incompatibility of many interspecific and inter-generic crosses, and therefore breeding for specific traits is often difficult. Sometimes, even though desirable genes (such as for cold hardiness, disease resistance, heat tolerance) are present in a closely related species, these are often linked to some other undesirable traits (poor propagation, weak growth habit) (Williams etal., 1990). In addition, the long generation cycle following hybridization delays rapid improvement. Therefore, combining several desirable traits such as cold hardiness, delayed senescence, and enhanced flowering, among others, into a single genotype is difficult and time consuming.
The techniques of genetic engineering can significantly speed up the progress of genetic improvement of rhododendrons and can circumvent the obstacles of traditional breeding methods. Transferring a desirable gene from a donor source (be it a related or unrelated species or genus) to a recipient elite genotype of rhododendron can be achieved without regard to sexual compatibility between donor and recipient genotypes, and without making crosses which result in random re-assortment of genes within a progeny. The latter phenomenon often results in the recovery of a different genotype than either parent used in a cross. However, using genetic engineering techniques to improve rhododendrons, or any other plant species, requires developing an effective means of transferring foreign DNA into a plant cell (a process termed "transformation") followed by developing a system to induce the transformed cell to divide and organize into a whole plant (a process termed "regeneration"). The regenerated plant (referred to as a "transgenic plant") may express the foreign DNA for a short duration, a phenomenon known as transient expression, or for the life of the plant, a phenomenon known as stable expression. Transient expression is useful to study how the foreign DNA or gene operates and functions in a recipient genotype; while stable expression is the desired goal for genetic engineering.
Developing a reliable and an efficient gene transfer system for rhododendrons is a prerequisite for developing transgenic plants. The two most commonly used methods for transformation of plants include Agrobacterium -mediated transfer (using a non-tumor inducing strain of the soil bacterium Agrobacterium tumefacines ) and microprojectile bombardment (using a gene gun) (Birch, 1997). Recently, Ueno etal. (1996) reported successful transformation of one cultivar of Rhododendron using Agrobacterium -mediated transfer and with a transformation efficiency of 5%. In our study, we wanted to utilize microprojectile bombardment as an alternative strategy for transformation of rhododendrons, as this method is easy to use and can possibly lead to a high efficiency of transformation (Potrykus, 1991; Birch, 1997). At present, several particle gene gun devices have been developed (Gray and Finer, 1993). The objectives of our study include developing an efficient direct DNA genetic transformation system for rhododendrons, and evaluating the reliability of the two most promising available gene gun devices.
Materials and Methods
PARTICLE GENE GUN DEVICES
Two different microprojectile devices were used for bombardment studies to compare the efficiency of these devices on genetic transformation of Rhododendron . The first device, a biolistic PDS-1000/He (Bio-Rad Laboratories, Richmond, California) particle delivery system, was used to deliver tungsten particles. This device was deployed by using a gap of 6 mm from rupture-disc to macrocarrier, a 6 mm macrocarrier flying distance, and a 9 cm stopping-screen to target distance. The second device, a home-made particle inflow gun (PIG), was built following the designs described by Finer etal. (1992) and Vain etal. (1993). The PIG was deployed using a distance of 11 or 13 cm from the syringe filter to target, helium tank pressure maintained at 60 psi with a pulse of 50 ms duration, and chamber pressure reduced to 28 mm Hg with a vacuum motor.
PLASMID AND DNA PREPARATIONS
The plasmid pZA300, consisting of a uidA reporter gene coding for ß-glucuronidase (GUS) and a hygromycin phosphotransferase (hpt) selectable marker gene, has been used for transformation in our study. Both genes are under the control of the cauliflower mosaic virus (CaMV)35S promoter and are followed by nopaline synthatase (NOS) polyadenylation sequences. The plasmid serves as a vector which allows cloning of genes in a particular order so they can be introduced into a plant cell where the genes can be expressed. The GUS gene is commonly used as a reporter gene to demonstrate transfer of a foreign gene into a host plant cell; while the htp gene is used for selecting plant cells that are transformed with a foreign gene, as they carry a gene for resistance against the antibiotic hygromycin and thus can grow on a selection medium containing this antibiotic.
The plasmid was isolated using the alkaline lysis method, and purified using a cesium chloride density gradient centrigfugation (Sambrook et al., 1989). Plasmid DNA was precipitated onto tungsten particles (average size of 1.1 µm) using a modification of the procedure described by Kikkert (1993). Two µ DNA (2.8 µg), 25 µl tungsten (1.5 mg), 25 µl 2.5M CaCl 2 , and 10µl 0.1M spermidine were mixed by continuous vortexing in a 0.6 ml microfuge tube, and then placed on ice for 10 minutes before centrifugation in an Eppendorf microfuge for a few seconds. After centrifugation, the supernatant was discarded, and the pellet was washed with 70% ethanol and centrifuged again for a few seconds. The supernatant was discarded and the pellet then resuspended in 10µl of 100% ethanol.
For bombardment using the PIG device, the DNA precipitation procedure was similar to the protocol described above, and 2 µl of the particle/DNA mixture were placed in the center of the syringe filter unit.
HISTOCHEMICAL ASSAY FOR CUS EXPRESSION
GUS assays were conducted 48 hours following bombardment using the histochemical assay of Jefferson (1987). The GUS assay buffer contained 0.5 mM potassium ferricyanide, 0.5 mM potassium ferrocyanide, 50 mM NaPC 4 , and 10 mM Na 2 EDTA. The 5-bromo-4-chloro-3-indoyl-ß-D-glucuronic acid (X-gluc) was dissolved in dimethyl formamide and added to the buffer at 0.05% w/v. Plant tissues were immersed into the GUS substrate mixture, and incubated for overnight at 37°C. In the presence of the GUS substrate, plant cells carrying the GUS reporter gene, transformed cells, would show blue stained spots. Data on number of ex-plants with blue spots were recorded 48 hours following bombardment. Percent explants with positive GUS expression (showing blue spots) was calculated and used for statistical analysis.
PLANT MATERIAL
Leaf sections, shoot-tips, and stem segments from in vitro-grown Rhododendron hybrids 'Hino-crimson' and 'Fuchsia' were used for microprojectile bombardment. Leaf sections were incubated on Anderson's medium (1984) containing 22.7 µM thidiazuron (TDZ) and 22.8 µM indoleacetic acid (IAA). Shoot-tips and stem segments were incubated on the same medium, but containing either 0.45 µM TDZ or 4.5 µM TDZ, respectively.
Shoot-tips, stem segments, and young fully-expanded leaves from in vitro-grown shoots were excised and pre-incubated for one week in the dark on the regeneration media described above. A Whatman No. 1 sterilized filter paper was used to cover the medium within each petri plate, and then the explant was placed onto the filter paper immediately prior to bombardment.
THE EFFECT OF HYGROMYCIN CONCENTRATIONS IN THE SELECTION MEDIUM ON SHOOT ORCANOCENESIS
Leaf sections of Rhododendron cv. 'Fuchsia' were incubated on a basal medium containing Anderson's (1984) salts, 4.54 µM TDZ, 0.29 µM IAA, and 87.6 mM sucrose. Hygromycin was filter-sterilized and added to the medium after autoclaving. The following concentrations of hygromycin were used: 0, 2, 4, 8, 16, 32, 64, and 128 mg•1 -1 . A total of three replications for each treatment was used. Each replicate consisted of 10 explants cultured in a 100 x 15 mm petri plate. Transformed plant cells therefore should be able to grow on a medium containing hygromycin as they would carry the hpt resistance gene, while non-transformed cells can not as they lack the hpt gene. Data on percent survival of leaf explants were collected after three weeks of culture in the dark.
THE INFLUENCE OF VARIOUS ACCELERATION PRESSURES ON GUS EXPRESSION
Shoot-tips, leaf sections, and stem segments of Rhododendron cv. 'Fuchsia' were pre-cultured for one week on the regeneration medium as described above, and then bombarded with tungsten microprojectiles using the biolistic PDS-1000/He gene gun. Each petri plate was bombarded twice using either 900, 1100, or 1300 psi rupture disks combined with a distance of 6 or 12 cm between the stopping screen and the target tissue. A total of two replications for each treatment was used. Each replicate consisted to 40 explants cultured in 100 x 15 mm petri plate. Data on percent transient GUS expression were collected after 48 hours after bombardment.
THE INFLUENCE OF OPEN-CHAMBER VS. PRE-CHAMBER COMBINED WITH DIFFERENT PHYSICAL DISTANCES FROM SYRINGE FILTER TO TARGET TISSUE ON TRANSFORMATION USING THE PIG DEVICE
The helium tank pressure of the PIG delivery system was set at 60 psi. An open-chamber bombardment involves leaving the needle valve which controls the flow of the helium pressure open, while a pre-chamber bombardment refers to filling the chamber first with helium and then closing the needle valve immediately prior to bombardment. In the latter case, the accelerating pressure used for bombardment corresponds to the helium pressure maintained within the chamber. Petri plates were placed on the adjustable shelf of the apparatus at distances of 13, 15, and 17 cm from the syringe filter unit. Each petri plate was bombarded twice and covered by a sterilized metal screen to protect the tissues from scattering.
Each treatment was replicated twice, and each replicate consisted of a single petri plate containing 40-50 calli induced from shoot-tips which were enough to cover a 4.25 cm diameter Whatman filter paper. Data on percent transient GUS expression were collected 48 hours after bombardment.
STATISTICAL ANALYSIS
All data were subjected to ANOVA; means were subjected to LSD tests at the 5% level using the SAS statistical analysis package. Linear and/or quadratic regression analysis were conducted using SAS's general linear model procedure (SAS Institute, Cary, N.C.). Simple linear or quadratic polynomial curves were fit to the data when significant trends were identified in the regression analysis.
Results and Discussion
THE EFFECT OF HYGROMYCIN CONCENTRATIONS IN THE SELECTION MEDIUM ON SHOOT ORGANOGENESIS
Determining the critical concentration of hygromycin in the selection medium is important, as we wish to identify cells carrying the hpt gene so they can be screened from non-transformed cells, but the antibiotic concentration used in the medium must not be too high as it can also prevent these transformed cells from growing or regenerating into shoots.
The growth of rhododendron leaf sections on media containing the antibiotic hygromycin at concentrations higher that 5 mg·l -1 was completely inhibited (Fig. 1). After 10 days of incubation, leaf sections grown on media containing >2.5 mg·l -1 hygromycin all turned dark-brown and eventually died. Although leaf sections on a medium containing 2.5 mg·l -1 remained alive, no callus development was observed. After four weeks of incubation in a 2.5 mg·l -1 hygromycin selection medium, no regeneration was observed (data not shown).
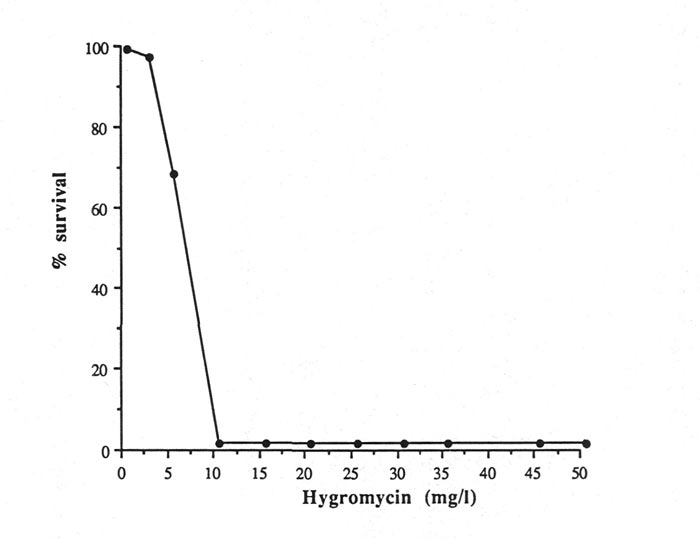
|
|
Fig. 1. Effect of hygromycin concentrations on survival of
Rhododendron hybrid 'Fuchsia' leaf explants after 2 weeks of culture on a selection medium. |
In contrast to rose embryogenic calli, rhododendron leaf sections are more sensitive to the presence of hygromycin in the medium. Vasil et al. (1993) reported that using a different selection agent, Basta (herbicide), instead of hygromycin in the selection medium induced different phenotypes of embryogenic calli. Since callus growth and regeneration of rhododendron leaf sections are completely inhibited at low concentrations of hygromycin, different types of antibiotics or different plant tissues which may have higher resistance to hygromycin should be considered for further studies.
THE INFLUENCE OF VARIOUS ACCELERATION PRESSURES ON CUS EXPRESSION
One case of transient GUS gene expression (a blue spot) was observed in a rhododendron leaf section subjected to an 1100 psi rupture disk with a 6 cm distance between the stopping screen and the target tissue. Therefore, this indicated that this one leaf section was transformed. No blue spots were found for any other accelerating setting for shoot-tips, calli, or stem segments (Table 1).
| Table 1. The effect of three accelerating pressures and two distance settings of the Biolistic PDS-1000/He gene gun on transformation of different tissues of Rhododendron hybrid 'Fuchsia'. | ||||
| Acceleration setting | % GUS expression ± SD | |||
| rupture disk (psi) | Distance* (cm) | shoot-tips | leaf sections | stem segments |
| 900 | 6 | 0±0 | 0±0 | 0±0 |
| 9 | 0±0 | 0±0 | 0±0 | |
| 1100 | 6 | 0±0 | 10±3 | 0±0 |
| 9 | 0±0 | 0±0 | 0±0 | |
| 1300 | 6 | 0±0 | 0±0 | 0±0 |
| 9 | 0±0 | 0±0 | 0±0 | |
| *: Distance from stopping screen to target explant. | ||||
A common range of acceleration parameters has been used for bombardment of rhododendron tissues in this experiment; however, low transient CUS expression was obtained. Since blue spots have been obtained on the control (tobacco leaves), DNA purity and the particle coating process were not limiting. Therefore, the physiological status of the plant cells/tissues might be responsible for this low transformation efficiency. Preculture of explants has been reported to enhance transformation efficiency in Douglas fir cotyledons (Goldfarb et al., 1991), and preconditioning of tissues by exposing them to auxin and cytokinin treatments increased the number of cells expressing the GUS gene. Using embryogenic callus of black spruce ( Picea mariana ), Duchesne and Charest (1991) reported that 5- to 6-day-old cells displayed a higher GUS expression than did younger or older cells. The influence of cell status on transformation was investigated by using synchronized cultured cells of tobacco (Lida et al., 1991). Their results indicated that cells bombarded at the M or G2 phases resulted in 4 to 6 higher transformation efficiency than those bombarded at the S or G1 phases. Therefore, a specialized regeneration protocol for different explant sources and a well defined cell-stage during the process of transformation and regeneration should be studied in order to enhance the transformation efficiency of Rhododendron.
THE INFLUENCE OF OPEN-CHAMBER VS. PRE-CHAMBER COMBINED WITH DIFFERENT PHYSICAL DISTANCE FROM TARCET TISSUE ON TRANSFORMATION USING THE PIG DEVICE
A 22.2% transient GUS gene expression was observed using an open-chamber setting at a 13 cm distance from the syringe filter to the shoot-tip derived calli of Rhododendron . An average of 1.5 blue spots for each responding callus was obtained (Table 2).
| Table 2. Effect of different chamber settings on transformation frequency of shoot-tip derived calli of Rhododendron hybrid 'Fuchsia' using the particle inflow gene gun. | ||
| % Transformed explants ± SD | ||
| Chamber setting | ||
| Distance* (cm) | pre-chamber | open-chamber |
| 13 | 0±0.0 | 22.2±9.8 |
| 15 | 0±0.0 | 0±0.0 |
| 17 | 0±0.0 | 0±0.0 |
| *: Distance from syringe filter to target explant. | ||
Approximately 500 ml of expanded helium was released at 1 atm. of the open-chamber, and using the pre-chamber this amount was reduced to 50 to 170 ml of expanded helium (Vain etal., 1993). Apparently, the higher helium setting promoted transient GUS expression; however, it was suggested that the pre-chamber might be valuable for stable transformation due to a reduction of damage to the target tissues (Vain etal., 1993). Since GUS expression of Rhododendron was low other factors for improving GUS expression should be investigated.
FUTURE DIRECTIONS
Although there are various gene transfer systems available now (Potrykus 1991; Birch, 1997), the barriers to obtaining transgenic plants are most likely due to lack of availability of reliable regeneration systems for various economically important crops (Hicks, 1994). Many successful regeneration systems for rhododendrons have been developed (Preece and Imel, 1991; Iapichino etal., 1992; Hsia and Korban, 1998), and transient gene expression has been obtained in this study. However, additional factors must be investigated to improve transformation protocols for rhododendrons in order to obtain stable transformants using microprojectile bombardment. Ueno et al. (1996) report on successful transformation of Rhododendron using Agrobacterium -mediated transfer and their recovery of stable transformants is a viable approach; however, this strategy needs to be tested and modified for many more cultivars and species of Rhododendron as well.
Efficient transient gene expression has been achieved in various species, but a low conversion rate of transient expression to stable transformation events has been observed (Hunold etal., 1995). Achieving stable transformation is still difficult; the stable-to-transient ratio was reported to be 1.9 x 10 -2 from tobacco leaves (Klein et al., 1988). Several physical factors for particle delivery such as the particle size and volume, particle coating procedure, DNA quantity and quality, depth of aperture of the barrel, must be taken into account (Sanford, 1993). However, there are a few other factors which may improve stable transformation. Mendel et al. (1989) have reported that large plasmids (12.8 and 14 kb) were less efficient than smaller plasmids suggesting that plasmid size is critical for stabilizing DNA within the cell.
The most commonly used marker gene, uidA for GUS expression, is assayed using a destructive method which limits the analysis. The use of anthocyanin marker genes coding for regulatory proteins of the structural genes of the anthocyanin biosynthetic pathway can be easily identified at the macroscopic level in living tissue while preserving the structure of the explant (Dupuis and Pace, 1993). Therefore, using marker genes for non-lethal histochemical assay should be considered to simplify the transformation protocols.
It has been suggested that using different promoters and plasmids may strongly increase transformation efficiency, and DNA constructs containing tissue-specific promoters can promote a higher level of gene expression and possibly lead to stable transformation (Duchesne and Charest, 1991). CaMV35S promoter, the most commonly used promoter, has been found to be poorly expressed in pollen and floral tissues (Franche et al., 1991). Similar observations have been reported by Twell etal. (1989), where they suggested that CaMV35S is developmentally-regulated in a cell-specific manner during microsporogenesis. A plasmid harboring a double CaMV35S, a stronger promoter, or a stress-inducible promoter, str 246C, which is induced in response to stress, can be induced following particle bombardment (Hunold etal., 1995).
Histological studies and knowledge of the physiological and morphological events during the process of regeneration can all help in improving the transformation process in plants. Sato etal. (1993) observed that the accelerated particles bombarded with the biolistic gun could penetrate more than two cell layers of a soybean shoot-tip. Based on cytological and histological observations of cotyledonary or immature zygotic embryos of sunflower, most tungsten particles did not penetrate farther than the cuticle, and only a few have been found in epidermal cells. In contrast, most gold particles were found within epidermal cells of leaves, and some were even detected in the palisade layer (Hunold etal., 1995). In our observations, both types of particles can be effective for transformation provided other parameters of bombardment are optimized. It has been suggested that the particle gene gun transformation system requires further development, and in particular a matching of regeneration potential with particle gun capability is key to developing efficient transformation protocols to develop transgenic plants.
Acknowledgment
This work was supported in part by a grant received from the American Rhododendron Society.
References
1. Anderson, W.C. 1984. A revised tissue culture medium for shoot multiplication of
rhododendron.
J. Amer. Soc. Hort.Sci.
109:343-347.
2. Birch, R.G. 1997. Plant transformation: problems and strategies for practical application.
Annu. Rev. Plant Physiol. Plant Mol. Biol.
48:297-326.
3. Duchesne, L.C. and Charest, P.J. 1991. Transient expression of the ß-glucuronidase gene
in embryogenic callus of
Picea mariana
following microprojection.
Plant Cell Rep.
10:191 -194.
4. Dupuis, I., and Pace, CM. 1993. Gene transfer to maize male reproductive structure by
particle bombardment of tassel primordia.
Plant Cell Rep.
12:607-611.
5. Finer, J.J., Vain, P., Jones, M.W., and McMullen, M.D. 1992. Development of the particle
inflow gun for DNA delivery to plant cells.
Plant Cell Rep.
11:323-328.
6. Franche, C., Bogusz, D., Schopke, C., Fauquet, C., and Beachy, R.N. 1991. Transient gene
expression in cassava using high-velocity microprojectiles.
Plant Mol. Biol.
17:493-498.
7. Goldfarb, G., Strauss, S.H., Howe, G.T., and Zaerr, J.B. 1991. Transient gene expression
of microprojectile-introduced DNA in Douglas-fir cotyledons.
Plant Cell Rep.
10:517-521.
8. Gray, D.J., and Finer, J.J. 1993. Development and operation of five guns for
introduction of DNA into plant cells.
Plant Cell Tiss. Org. Cult.
33:219.
9. Hicks, G.S. 1994. Shoot induction and organogenesis in vitro: a developmental perspective.
In Vitro Cell Dev. Biol.
30:10-15.
10. Hsia, C.-N, and Korban, S.S. 1998. Effect of growth regulators, dark treatment, and
light intensity on shoot organogenesis from leaf tissues of evergreen azalea.
J. Hort. Sci.
Biotech.
73:53-60.
11. Hunold, R., Burrus, M., Bronner, R., Duret, J-P, and Hahne, G. 1995. Transient gene
expression in sunflower (
Helianthus annuus
L.) following microprojectile bombardment.
PlantSci.
105:95-109.
12. Iapichino, G., McCullock, S., and Chen, T.T.T. 1992. Adventitious shoot formation
from leaf explants of rhododendron.
Plant Cell Tiss. Org. Cult.
30:237-241.
13. Jefferson, R.A. 1987. Assaying chimeric genes in plants: the GUS gene fusion system.
Plant Mol. Biol. Rep.
5:387-405.
14. Kikkert,J.R. 1993. Thebiolistic PDS-1000/He device.
Plant Cell Tiss. Org. Cult.
33:221-226.
15. Klein, T.M., Harper, E.C., Svab, Z., Sanford, J.C., Fromm, M.E., and Maliga, P. 1988.
Stable genetic transformation of intact
Nicotiana
cells by the particle bombardment
process.
Proc. Natl. Acad. Sci.
85:8502-8505.
16. Lida, A., Yamashita, T., Yamada, Y., and Morikawa, H. 1991. Efficiency of
particle-bombardment-mediated transformation in influences by cell cycle stage in synchronized
cultured cells of tabacco.
Plant Physiol.
97:1585-1587.
17. Mendel, R.R., Muller, B., Schulze, J., Kolesnikov, V., and Zelenin, A. 1989. Delivery
of foreign genes to intact barley cells by high-velocity microprojectiles.
Theor. Appl. Genet.
78:31-34.
18. Potrykus, I. 1991. Gene transfer to plants: assessment of published approaches and results.
Annu. Rev. Plant Physiol. Plant Mol. Biol.
42:205-225.
19. Preece, J.E., and Imel, M.R. 1991. Plant regeneration from leaf ex-plants of
Rhododendron
'P.J.M. Hybrid'
Scientia Hort.
48:159-170.
20. Sambrook, J., Fritsch, E.F., and Maniatis, T. 1982. Molecular cloning, a laboratory
manual. Cold Spring Harbour Lab., New York.
21. Sanford, J.C. 1990. Biolistic plant transformation.
Physiol. Plant.
79:206-209.
22. Sanford, J.C, Smith, F.D., and Russell, J.A. 1993. Optimizing the biolistic
process for different biological applications.
Methods in Enzymology
217:483-509.
23. Sato, S., Newell, C., Kolacz, K., Tredo, L., Finer, J., and Hinchee, M. 1993.
Stable transformation via particle bombardment in two different soybean regeneration system.
Plant Cell Rep.
12:408-413.
24. Twell, D., Klein, T.M., Fromm, M.E., and McCormick, S. 1989. Transient expression
of chimeric genes delivered into pollen by microprojectile bombardment.
Plant Physiol.
91:1270-1274.
25. Ueno, K., Fugkunaga, Y., and Arisumi, K. 1996. Genetic transformation of
Rhododendron
by
Agrobacterium
tumefaciens.
Plant Cell Rep.
16:38-41.
26. Vain, P., Keen, N., Murillo, J., Rathus, C, Nemes, C, and Finer, J.J. 1993.
Development of particle inflow gun.
Plant Cell Tiss. Org. Cult.
33:237-246.
27. Vain, P., McMullen, M.D., and Finer, J.J. 1993. Osmotic treatment enhances
particle bombardment-mediated transient and stable transformation of maize.
Plant Cell
Rep.
12:84-88.
28. Vasil,V., Srivastava,V., Castillo, A.M., Fromm, M.E., and Vasil, I.K. 1993.
Rapid production of transgenic wheat plants by direct bombardment of cultured immature
embryos.
Bio/Technology
. 11:1553-1558.
29. Williams, E.G., Rouse, J.L., Palser, B.F., and Knox, R.B. 1990. Reproductive
biology of
Rhododendron. Hort Rev.
12:1-67.
