JARS v57n1 - A Unique ''Giant Cell'' Type in Leaves of Vireyas
A Unique 'Giant Cell' Type in Leaves of Vireyas
Erik T. Nilsen and Stephen E. Scheckler
Biology Department
Virginia Polytechnic Institute and State
University
Blacksburg, Virginia
Synopsis
Giant cells called idioblasts were found in and below the epidermis in many vireyas. Idioblasts may have taxonomic significance because they were not found in many nonvireyas. Different vireya species have different sizes and abundances of idioblast cells. Idioblasts may function in water relations, light absorption, secretion, or defense.
One of the most fascinating aspects of Rhododendron is its diversity of species and habitats where those species grow. Studying species of Rhododendron and their ecology will help us improve our horticultural acumen with them as well as increase our knowledge of plant ecology in general. In fact, Rhododendron may serve as an excellent model for understanding the adaptive significance of plant traits to the fitness of those plants across broad regional climates.
The genus Rhododendron now contains over 1000 species, found in all continents of the northern hemisphere (Chamberlain et al. 1996). These species are grouped into eight major subgenera, within which 12 sections and 59 subsections are found. Most species are grouped into the subgenera Hymenanthes (302 species in 24 subsections) and Rhododendron (541 species in 34 subsections). Section Vireya has the largest number of species (310) in comparison to all other sections of the genus.
During a larger study to determine if leaf morphological and anatomical traits are similarly correlated with broad latitude gradients in three continents (Asia, North America, and Europe), it became clear that the leaf anatomy of Vireya species was different from species in other sections of the genus Rhododendron because of the presence of "giant cells" (idioblasts). Based on this observation, we felt it prudent to digress on our research track to describe this unusual anatomy and evaluate if these characters are restricted to Vireya and universal among subsections of Vireya . Based on the anatomy of Vireya leaves and the variation in anatomical traits among different species we speculate about the ecological and physiological significance of idioblasts to Vireya species.
Species Selection Criteria
All the material used in this study was collected at the Rhododendron Species Foundation/Botanical Garden (RSF) in Federal Way, Washington, USA. We selected this one source to insure that environmental effects on leaf anatomical traits were minimized. Thus, the RSF served as a common garden for our research program. This location was also selected because the RSF maintains a living collection of approximately 400 species of Rhododendron and the RSF goes to great lengths to assure that the plants in their collection represent genotypes from the natural distribution of each species (Barrett 1994). Twenty six species within Vireya , encompassing all seven subsections of Vireya , were selected for analysis (Table 1). Also, 27 species were selected from other sections in the subgenus Rhododendron (which includes the section Vireya ) and Hymenanthes in order to provide a preliminary comparison with our results from members of the section Vireya (Table 1). All Vireya species were grown inside greenhouses because of their vulnerability to freezing in the Federal Way region. Several of the species selected from other parts of the genus were also grown in RSF greenhouses.
| Table 1: Species of Rhododendron and their taxonomic designation (based on Chamberlain et al. 1996) utilized in the study of leaf anatomy of the section Vireya . Species are listed alphabetically within subsections. | ||||
| Species | Authority | Subgenus | Section | Subsection |
| arboreum ssp. cinnamomeum | Sm. (1805) | Hymenanthes | Ponticum | Arborea |
| adenopodum | Franch (1895) | Hymenanthes | Ponticum | Argyrophylla |
| barbatum | Wall. ex G. Don (1834) | Hymenanthes | Ponticum | Barbata |
| sinogrande | Balf.f. & W.W. Sm. (1916) | Hymenanthes | Ponticum | Grandia |
| tsariense | Cowan (1937) | Hymenanthes | Ponticum | Lanata |
| pachysanthum | Hayata (1913) | Hymenanthes | Ponticum | Maculifera |
| aureum | Georgi (1794) | Hymenanthes | Ponticum | Pontica |
| brachycarpum | D. Don ex G. Don (1834) | Hymenanthes | Ponticum | Pontica |
| catawbiense | Michx. (1803) | Hymenanthes | Ponticum | Pontica |
| caucasicum | Pall. (1788) | Hymenanthes | Ponticum | Pontica |
| degronianum | Carriere (1869) | Hymenanthes | Ponticum | Pontica |
| degronianum ssp. yakushimanum | (Nakai) H. Hara (1986) | Hymenanthes | Ponticum | Pontica |
| hyperythrum | Hayata (1913) | Hymenanthes | Ponticum | Pontica |
| macrophyllum | D. Don ex G. Don (1834) | Hymenanthes | Ponticum | Pontica |
| makinoi | Tagg (1927) | Hymenanthes | Ponticum | Pontica |
| maximum | L. (1753) | Hymenanthes | Ponticum | Pontica |
| ponticum | L. (1762) | Hymenanthes | Ponticum | Pontica |
| smirnowii | Trautv. (1885) | Hymenanthes | Ponticum | Pontica |
| ungernii | Trautv. (1885) | Hymenanthes | Ponticum | Pontica |
| williamsianum | Rehder & E.H. Wilson (1913) | Hymenanthes | Ponticum | Williamsiana |
| carolinianum | Rehder (1912) = R. minus | Rhododendron | Rhododendron | Caroliniana |
| minus var. chapmanii | (A.Gray) Duncan & Pullen (1962) | Rhododendron | Rhododendron | Caroliniana |
| minus var. minus | Michx. (1792) | Rhododendron | Rhododendron | Caroliniana |
| lapponicum | (L.) Wahlenb.(1812) | Rhododendron | Rhododendron | Lapponica |
| groenlandicum | (Oeder) Kron & Judd (1990) | Rhododendron | Rhododendron | Ledum |
| ferrugineum | L. (1753) | Rhododendron | Rhododendron | Rhododendron |
| hanceanum | Hemsl. (1889) | Rhododendron | Rhododendron | Tephropepla |
| correoides | J.J.Sm. (1915) | Rhododendron | Vireya | Albovireya |
| malayanum | Jack (1822) | Rhododendron | Vireya | Malayovireya |
| bryophilum | Sleumer | Rhododendron | Vireya | Phaeovireya |
| konori | Becc. (1878) | Rhododendron | Vireya | Phaeovireya |
| rarum | Schltr. (1918) | Rhododendron | Vireya | Phaeovireya |
| superbum | Sleumer (1960) | Rhododendron | Vireya | Phaeovireya |
| kawakami | Hayata (1911) | Rhododendron | Vireya | Pseudovireya |
| sororium | Sleumer (1958) | Rhododendron | Vireya | Pseudovireya |
| herzogii | Warb. (1892) | Rhododendron | Vireya | Siphonovireya |
| loranthiflorum | Sleumer (1935) | Rhododendron | Vireya | Solenovireya |
| maius | (J. J. Sm.) Sleumer(1960) | Rhododendron | Vireya | Solenovireya |
| aurigeranum | Sleumer (1960) | Rhododendron | Vireya | Vireya |
| blackii | Sleumer (1973) | Rhododendron | Vireya | Vireya |
| burtii | P. Woods (1978) | Rhododendron | Vireya | Vireya |
| elebicum | (Blume) DC. (1839) | Rhododendron | Vireya | Vireya |
| crassifolium | Stapf (1894) | Rhododendron | Vireya | Vireya |
| gracilentum | F. Muell. (1889) | Rhododendron | Vireya | Vireya |
| lochiae | F. Muell. (1887) | Rhododendron | Vireya | Vireya |
| longiflorum | Lindl. (1848) | Rhododendron | Vireya | Vireya |
| javanicum | (Blume) Benn (1838) | Rhododendron | Vireya | Vireya |
| macgregoriae | F. Muell. (1891) | Rhododendron | Vireya | Vireya |
| polyanthemum | Sleumer (1963) | Rhododendron | Vireya | Vireya |
| praetervisum | Sleumer (1973) | Rhododendron | Vireya | Vireya |
| rugosum | Low ex Hook. f. (1852) | Rhododendron | Vireya | Vireya |
| stenophyllum | Hook. f. ex Stapf (1878) | Rhododendron | Vireya | Vireya |
| zoelleri | Warb. (1892) | Rhododendron | Vireya | Vireya |
Leaf Sample Preparation
Two leaves were collected from each of five individuals for each species. Only fully mature leaves from the current growing season on outer canopy branches were selected in order to insure similarity of developmental stage among species. Morphology measurements (length, width area) were taken on all leaves (n=10/species). One leaf from each plant was randomly selected (coin flip) for anatomical measurements. At the midsection of each leaf a rectangular piece was excised that extended from the leaf margin to the midvein. These pieces were immediately preserved in a fixative (Formalin, acetic acid and alcohol). After complete fixation the samples were dehydrated and embedded in paraffin blocks. Sections (10 micron thickness) were adhered to microscope slides and stained with Safranin-O and Fast Green. This technique was selected to highlight the presence of secondary with Safranin O, and primary cell wall structure with Fast Green (Ruzin 1999).
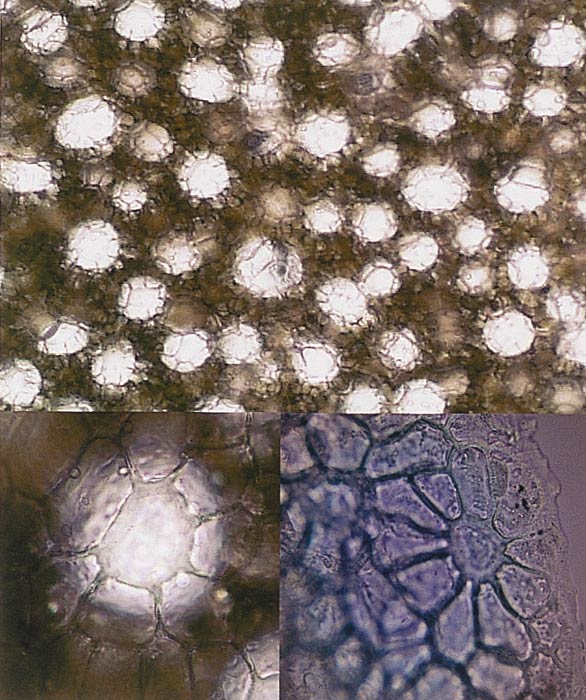
|
|
The top image is a photomicrograph of a free hand
paradermal section through a leaf of
R. zoelleri . Each light patch, surrounded by a ring of epidermal cells, represents an individual idioblast "Giant Cell." The lower left hand photomicrograph is a higher magnificationof one idioblast of a R. zoelleri leaf and its surrounding ring of epidermal cells. The bottom right photograph is taken at an oblique angle to show the three-dimensional nature of the epidermal cells around the idioblast on a R. zoelleri leaf. Photo by author |
Leaf Anatomical Characters
Anatomical traits were measured on each microscope slide. Leaf thickness, thickness of the palisade mesophyll, and thickness of the spongy mesophyll were measured on photomicrographs of each slide. Photomicrographs were captured with a digital camera (Olympus model DP-10) mounted on a bright field microscope (Olympus model BX50). Measurements were made on these images (and images of a stage micrometer) with the use of an image analysis program (SCION image, Scion Corporation, Fredrick, Maryland). The volume of inflated cells (termed idioblasts) found in the epidermis of Vireyas was determined by measuring the width and length of 10 cells per slide. The average length and width was used to calculate mean inflated cell volume by using the geometric formula for an oblate spheroid (4/3 πa2b), where a = major semiaxis and b = minor semiaxis (Selby 1972).
| Table 2. Anatomical characteristics of leaves from Rhododendron section Vireya plants grown at the Rhododendron Species Foundation/Botanical Garden, Federal Way, Washington. Species are listed alphabetically. Numbers in parentheses are standard deviations. Pal/Spo = the thickness of the palisade mesophyll divided by the thickness of the spongy mesophyll. N = 5 (1 leaf from each of 5 individuals). | ||||
| Species | Distended Cell | epidermis | palisade | Pal / Spo |
| volume (mm 3 X 10 -5 ) | # of layers | # of layers | (mm / mm) | |
| aurigeraneum | 0.50 (0.14) | 1 | 2.5 | 0.78 (0.23) |
| blackii | 1.56 (0.14) | 1 | 2.5 | 0.79 (0.24) |
| bryophilum | 3.90 (0.02) | 1 | 1.5 | 1.21 (0.15) |
| burtii | 2.15 (1.30) | 2 | 2.5 | 0.72 (0.23) |
| celebicum | 1.07 (0.38) | 1 | 2.5 | 0.97 (0.18) |
| correoides | 0.67 (0.42) | 1 | 2.5 | 0.77 (0.18) |
| crassifolium | 2.95 (0.89) | 1 | 1.5 | 0.67 (0.22) |
| gracilentum | 3.29 (0.24) | 1 | 2 | 0.77 (0.23) |
| herzogii | 2.52 (2.44) | 1 | 2 | 0.85 (0.24) |
| javanicum | 2.44 (1.33) | 1 | 2.8 | 1.22 (0.14) |
| kawakami | 3.30 (3.01) | 1 | 3.5 | 1.08 (0.06) |
| konori | 0.61 (0.26) | 1 | 3.5 | 0.80 (0.08) |
| lochiae | 0.74 (0.66) | 1 | 3.5 | 1.03 (0.24) |
| longiflorum | 3.34 (2.27) | 1 | 2 | 1.03 (0.24) |
| loranthiflorum | 3.01 (0.210) | 1 | 2.5 | 1.12 (0.44) |
| macgregoriae | 1.45 (0.99) | 1 | 2.5 | 0.92 (0.09) |
| maius | 1.58 (0.26) | 2 | 2.5 | 0.89 (0.35) |
| malayanum | 5.96 (0.30) | 2 | 2 | 0.93 (0.30) |
| polyanthemum | 1.50 (0.10) | 1 | 3.5 | 0.50 (0.24) |
| praetervisum | 3.82 (1.96) | 2 | 2.5 | 1.04 (0.16) |
| rarum | 3.63 (0.94) | 1 | 1.5 | 1.22 (0.15) |
| rugosum | 0.54 (0.14) | 2 | 1.5 | 0.78 (0.34) |
| sororium | 2.17 (1.30) | 1 | 1.8 | 0.89 (0.16) |
| stenophyllum | 2.78 (0.85) | 1 | 1.5 | 0.77 (0.29) |
| superbum | 1.03 (0.44) | 1 | 3.5 | 0.71 (0.149) |
| zoelleri | 3.53 (2.00) | 1 | 3.5 | 1.02 (0.134) |
The mean ratio of palisade to spongy mesophyll thickness was not significantly different between the leaves of the selected Vireya and non- Vireya species (0.88 � 0.05 and 0.99 � 0.04 respectively). Moreover, the distribution of this anatomical trait among the species within each group was similar (Figure 1D). There was no significant difference in the average number of palisade cell layers in leaves of the selected Vireya and non- Vireya species (2.82 � 0.65 and 3.06 � 0.45 respectively). Also, the selected Vireya species had a higher proportion of leaves with 1-3 palisade cell layers than those of the selected non- Vireya species (Figure 1E). Stomatal frequency varied from 105 to 665 per mm 2 among species and the mean stomatal frequency for the selected Vireya species was lower but not significantly different from the selected non- Vireya species (250 � 125 and 419 � 187 respectively). The selected Vireya group tended to have a higher proportion of species with fewer stomata per area than the selected non- Vireya group (Figure 1F). All other anatomical traits measured for these two groups of species were very similar except for the presence of unusually large sub-epidermal cells.
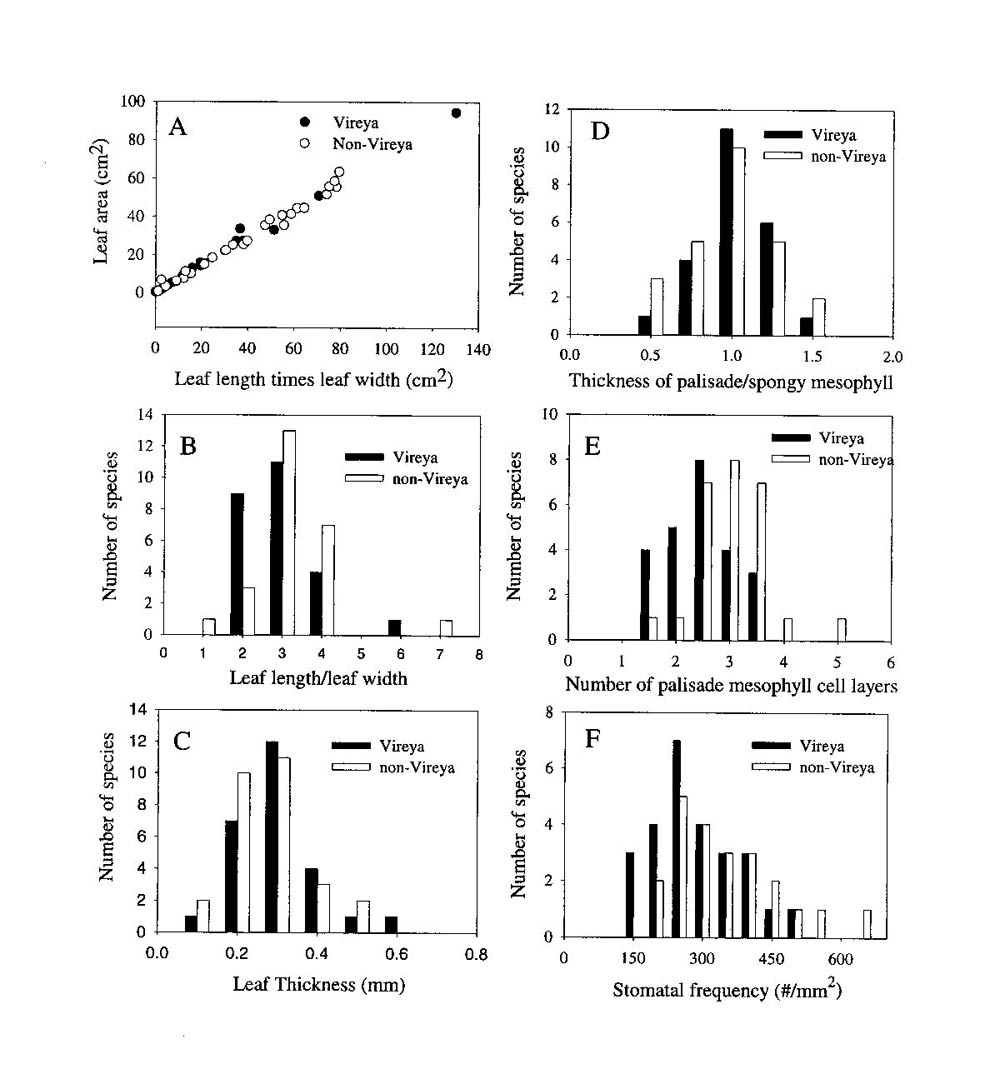
|
|
Figure 1. A comparison of morphological and anatomical traits between a selected group of
Rhododendron
species from the section
Vireya
(n = 26) and a selected group of Rhododendron species from other sections and subgenera of Rhododendron (n = 27). All species values were formulated for mean values per species (n = 5 plants per species). All Rhododendron species were grown at the Rhododendron Species Foundation/Botanical Garden in Federal Way, Washington, USA. |
All Vireya species selected for this study were characterized by the presence of large cells associated with the epidermis (Figure 2). Similar large cells associated with the epidermis have been described for several dicot angiosperms and are called idioblasts (e.g., Lersten and Curtis 1995). The idioblasts we analyzed in Vireya species were always associated with the epidermis but extended to the leaf surface in some species (e.g., R. zoelleri Fig 2) or resided below a single or multiple epidermal layer in other species. None of the selected non- Vireya species contained this cell type. Although 100% of our sampled Vireya species had these large idioblasts, the size and distribution of the cells varied among species. The average volume of the idioblast cells ranged from 0.50 � 0.14 to 5.96 � 0.30 x 10-5 mm 3 with a mean of 2.31 � 1.32 x 10-5 mm 3 . In some species (e.g., R. bryophilum ), these cells occupied as much as 25% of the total leaf volume (Figure 2). Although these cells were always located in or under the adaxial (top) leaf surface, in some species they also were located on the abaxial surface. The inflated cells on the abaxial (bottom) side were always smaller than those on the adaxial leaf surface of the same species. Moreover, there was a wide variation in the abundance of these cells on the adaxial (top) side. Abundance of inflated cells varied from a continuous layer to infrequent. On the abaxial side the smaller, inflated cells were often bunched into groups and frequently associated with the base of an epidermal scale.
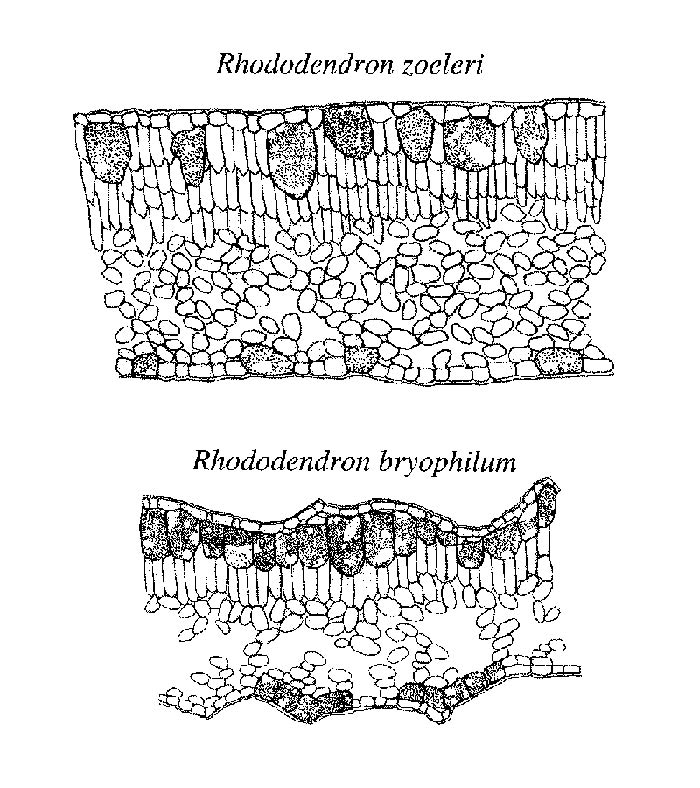
|
|
Figure 2. Diagrammatic representations of leaf cross sections of two
Vireya
species.
Artistic rendering by Lara Call |
The interior of the idioblasts retained Safranin-O stain, which is in contrast to the other epidermal cells. Palisade cells retained Safranin-O stain only in chloroplasts while the idioblasts retained the stain throughout the cell volume. Bright field microscopy indicated that the inflated cells contained vesicles, droplets and fibrous material in the large central vacuole (Figure 3A).
Electron Microscopy
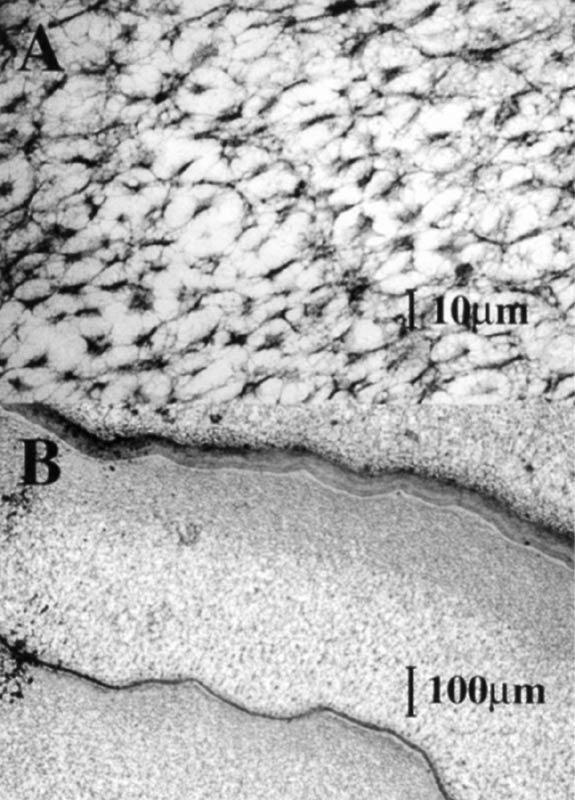
Samples from selected Vireya species were prepared for transmission electron microscopy (TEM) to determine the structure of the material in the central vacuoles of the idioblasts. Fresh leaf tissues were fixed in a gluteraldehyde fixative (5% gluteraldehyde, 4.4% formaldehyde, 2.75% picric acid, in 0.05% Na cacodylate) and then prepared for sectioning with standard procedures. Samples were sectioned on an ultramicrotome, placed on grids and observed with a JOEL electron microscope. Samples from the selected Vireya species also were prepared for scanning electron microscopy (SEM) to study the adaxial (top) surface cell architecture. Wax coatings on the top surface precluded any clear observations of epidermal cell architecture. Here we present only one image taken of the cut edge of one species.
The SEM images indicated that there were structural materials in the central vacuoles of the idioblasts (Figure 3B). The TEM images demonstrated that the vacuolar material of some Vireya species was sponge-like (Figure 4A). Moreover, membranous separations were found in the central vacuoles (Figure 4B), which suggest that in some cases the idioblasts may be a product of several cells formed without a cell wall between them. Dense cytoplasm that contained nuclei and other organelles was found at the edges of the idioblasts, which indicates that they are living functional cells.

|
|
Figure 3. A: A bright field photomicrograph of several idioblasts in a
hand cross section of unstained fresh leaf material of Rhododendron zoelleri . B: Scanning electron micrograph (SEM) image of idioblasts in a leaf of Rhododendron javanicum . |
Systematic Significance of Idioblasts in Vireya Species
Sleumer (1980) stated that he had "looked in vain for really constant characters that could distinguish Vireya from the rest of the subgenus Rhododendron ." Two characters (tailed seeds, and twisted valves after capsule opening) were eventually selected which could only be used in a general sense. Our data indicate that the presence of idioblasts associated with the adaxial (top) epidermis may be a universal characteristic of the section Vireya . It was the only leaf trait that we found to vary significantly between our selected groups of Vireya and non- Vireya species. We found these idioblasts in 100% of our sampled Vireya species, which included members from all seven currently identified subsections and from most of the natural geographic range of the section Vireya . Of course, our sample of 26 species is only a small fraction of the 300+ species of Vireya , but the 100% occurrence rate suggests an excellent taxonomic association. Moreover, these results suggest that Vireya is a good monophyletic group in Rhododendron , and that the presence of idioblasts is an ancient trait for this group. Recent studies of two gene sequences also indicated that Vireya is a monophyletic group in Rhododendron (Kurashige et al. 2001). Continued examination of this trait, in a wider diversity of species in the subgenera Rhododendron and Hymenanthes , would greatly strengthen the value of this taxonomic character, especially if it proves to be a synapomorphy (universally found derived character) for the entire Vireya section.
|
|
Figure 4. A. Transmission electron micrograph (TEM) image of the matrix within
the central vacuole of idioblasts in a leaf of Rhododendron sororium . B. Transmission electron micrograph of membranous materials traversing the central vacuole of idioblasts in a leaf of Rhododendron sororium . |
Ecological Significance of Idioblasts in Vireya Species
The extent of variation in cell volume, abundance, and internal distribution suggests that there are ecological or physiological significances of the idioblasts. They are not simply a vestigial remnant of a past eco-physiological history. In the following we consider the evidence for several different possible roles of idioblasts to Vireya leaf physiology and ecology.
Some idioblasts could also serve as a lens to refract light entering leaves and increase leaf absorptance in the palisade mesophyll where most photosynthesis occurs. Light penetrating into the idioblasts may ricochet off the vesicles, membranes, and matrix in the central vacuole causing the photons to pass through a thicker layer of palisade cells before exiting the leaf than they would without the idioblasts. We have taken measurements of leaf absorptive properties of Vireya and non- Vireya species (of similar leaf thickness). Those measurements show that leaf absorptance and transmittance characteristics are similar for Vireya and non- Vireya species. Although the presence of idioblasts did not have a significant effect on whole leaf absorptance, they may alter the pattern of light penetration into the leaf interior creating specific regions of high illumination. Micro measurement of light penetration patterns (e.g., Vogelman et al. 1996) into leaves of Vireya species would help us to determine the significance of idioblasts to light penetration patterns.
Other idioblasts could serve as a mechanism for leaf curling in Vireya . Large expanded epidermal cells in corn leaves (called bulliform cells) are responsible for corn leaf curling. In the case of Vireya leaves, idioblasts could expand and shrink in response to water availability if their cell walls were flexible enough. Since the idioblasts are mostly on the adaxial surface, shrinkage in volume should cause leaf curling upward. Although leaf curling is well known in Rhododendron (Nilsen 1992) and occurs in leaves from plants in both the Hymenanthes and Rhododendron subgenera (Nilsen 1991), it usually occurs in a downward direction. Moreover, many species that express extreme leaf curing in Rhododendron do not have idioblasts (e.g., R. catawbiense , R. maximum ), and none of the Vireya species in this study demonstrated any amount of curling in response to freezing or desiccation. These observations clearly do not support idioblast function for regulating leaf curling in Vireya .
Another possibility is that idioblasts serve as a water storage system (a type of succulence). The sponge-like ultrasturucture of the material in the vacuole may serve as a mechanism to hold large quantities of water. Also, we observed that the most succulent Vireya leaves (thickest leaves with the smallest intercellular air spaces) had the smallest and least frequent idioblasts (e.g., R. polyanthemum ). This observation agrees with the possible water storage significance because idioblasts would have little importance in leaves that for other reasons are already succulent. Research on the water relations of leaves with varying amounts of idioblasts is necessary to verify their water storage capacity.
The idioblasts could also serve as a gland for exuding materials onto the leaf surface. This possibility was supported by the arrangement of cells in the adaxial epidermis of R. zoelleri (Figure 4). The apex of each inflated cell extends to the leaf surface and is surrounded by a ring of epidermal cells. In addition, TEM images (not shown here) suggest that material oozes out to the epidermis from the region between the upper edges of the inflated cells and the adjacent epidermal cells. Moreover, epidermal cells surrounding the apex of the idioblasts were connected through highly modified thin regions of cell wall (Figure 5). This specific epidermal cell arrangement and TEM ultrastructure is characteristic of epidermal glands, but was found only for leaves of R. zoelleri . Idioblasts may have a glandular function in some species but not others.
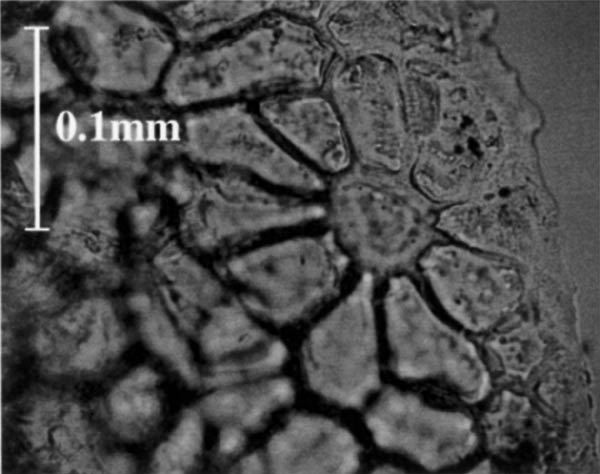
|
|
Figure 5. A bright field photomicrograph (oblique paradermal view) of an
unstained hand section of the adaxial (top) surface of fresh leaf material of Rhododendron zoelleri . Notice the ring of epidermal cells surrounding the central area, which is the apex of an idioblast. |
Safranin-O was retained in the vacuole of idioblasts in all but one Vireya species studied. Safranin-O preferentially stains nucleoli, chromosomes, lignified cell walls, mucilage, chloroplasts, cutin, chitin, and suberin (Ruzin 1999). The sponge-like material in the vacuole of the idioblasts of R. sororium (Figure 4A) could be composed of polymerized materials such as mucilage, cutin, protein, etc. The presence of these polymers may act as a herbivore defense mechanism, as has been found for other species (Orcutt and Nilsen 2000, chapter 7). When an insect chews into the Vireya leaf, many idioblasts would rupture and the vacuolar contents would leak out and interfere with feeding or be toxic. Insect feeding trials with Vireya leaves are needed to evaluate the significance of the idioblasts to herbivore defense.
Summary
Most morphological and anatomical traits of the selected Vireya and non- Vireya species examined were not significantly different. However, the presence of idioblasts was unique to species in samples tested for the section Vireya . All of the Vireya species we selected had this trait and our selection included members from all taxonomic subsections and a broad range of native geographic regions occupied by species of the section Vireya . This trait may be a valuable taxonomic indicator of the section Vireya , and deserves to be examined in a wider number of species. The abundance, size, and ultrastructure of idioblasts differs among Vireya species, which suggests that the ecological or physiological significance of idioblasts differs among Vireya species. Most likely these idioblasts function in leaf water balance, glandular secretion, light penetration, or herbivore defense. Further research may underline the importance of this taxonomic trait and could help to explain the evolution of this important tropical section of the genus Rhododendron .
Acknowledgements
The authors thank Darren DeStefano, Keli Goodman, Brian Hacker, Tina Keesee, Andrea Venetz, and Amber Waller for help with sample preparation and photomicrography. We wish to thank Lara Call for drawing Figure 2. Also, many thanks go to Rick Peterson (Co-Executive Director of the Rhododendron Species Foundation and Botanical Garden, Federal Way, Washington) for many hours of help collecting leaf samples and helping with logistics at the research garden. We appreciate the help we received with electron microscopy from Kathy Lowe and Virginia Viers at the Morphology Laboratory of the Virginia-Maryland College of Veterinary Medicine. This research was supported by funds from the Virginia Tech College of Arts and Sciences Millennium program.
References
1. Barrett C. 1994.
History of the Rhododendron Species Foundation. Genesis of a Botanical Garden
.
Positive Attitudes, Publishers Eugene, OR.
2. Blume C.L. 1826. Bijdarden tot de flora van Nederlandsch Indif. Batavia.
3. Chamberlain D. 1982. A revision of Rhododendron. II. Subgenus
Hymenanthes
.
Notes of the
Royal Botanical Garden Edinburgh
39: 209-486.
4. Chamberlain, D., R. Hyam, G. Argent, G. Fairweather, and K.S. Walter. 1996.
The Genus Rhododendron.
Its classification & synonymy
. Royal Botanical Garden, Edinburgh, U.K.
5. Chamberlain D., and S. J. Rae. 1990. A revision of
Rhododendron
. IV. Subgenus
Tsutsusi
.
Edinburgh Journal of Botany
47: 89-200.
6. Cullen J. 1980. A revision of
Rhododendron
. I. Subgenus
Rhododendron
sections
Rhododendron
and
Pogonanthum
.
Notes of the Royal Botanical Garden
Edinburgh
39: 1-207.
7. Don G. 1834.
A General History of Dichlamydeous Plants
. J.F. and F. Rivington Publishers,
London.
8. Kurashige Y., J.L. Etoh, T. Handa, K. Takayanagi, and T. Yukawa. 2001. Sectional relationships in
the genus
Rhododendron
(
Ericaceae
): evidence from matK and trnK intron sequences.
Plant Systematics and Evolution
228: 1-14.
9. Lersten, N.T., and J.D. Curtis. 1995. Two foliar idioblasts of taxonomic significance in Cercidium
and Parkensonia (Leguminosae: Ceasapinioideae).
American Journal of Botany
82: 565-570.
10. Lipp C.C., and E. T. Nilsen. 1997. The impact of sub-canopy light environment on the hydraulic
vulnerability of
Rhododendron maximum
, to freeze-thaw and drought.
Plant Cell and
Environment
. 20: 1264-1272.
11. Linnaeus C. 1753.
Species Plantarum
. Edition 1. Stockholm.
12. Nilsen, E.T. 1985. Seasonal and diurnal leaf movements of
Rhododendron maximum
L.
in contrasting irradiance environments.
Oecologia
65: 296-302.
13. Nilsen, E.T. 1986. Quantitative phenology and leaf survivorship of
Rhododendron maximum
L. in contrasting irradiance environments of the Appalachian mountains.
American Journal of
Botany
73: 822-831.
14. Nilsen, E.T. 1991. The relationship between freezing tolerance and thermotropic leaf movement
in five
Rhododendron
species.
Oecologia
87: 63-71.
15. Nilsen, E.T. 1992. Thermonastic leaf movements: A synthesis of research with
Rhododendron
.
The Botanical Journal of the Linnean Society
110: 205-233.
16. Nilsen, E.T., and Y. Bao. 1987. The influence of age, season, and microclimate on the
photochemistry of
Rhododendron maximum
. I: Chlorophylls.
Photosynthetica
1: 535-542.
17. Nilsen, E.T., D.A. Stetler, and C. A. Gassman. 1988. The influence of age and microclimate
on the photochemistry of
Rhododendron maximum
leaves II . Chloroplast structure
and photosynthetic light response.
American Journal of Botany
75: 1526-1534.
18. Orcutt, D.M., and E.T. Nilsen. 2000.
The Physiology of Plants Under Stress: Soil and Biotic
Factors
. John Wiley Incorporated, New York.
19. Philipson W.R., and M. N. Philipson. 1986. A revision of
Rhododendron
. III. Subgenera
Azaleastrum
,
Mumeazalea
,
Candidastrum
and
Therorhodion
.
Notes of the
Royal Botanical Garden Edinburgh
44: 1-23.
20. Ruzin, S.E. 1999.
Plant Microtechnique and Microscopy
. Oxford University Press, New York.
21. Selby, S.M. 1972.
Standard Mathematical Tables, Twentieth Edition
. CRC Press, Cleveland,
Ohio.
22. Sleumer, H.O. 1966.
An account of Rhododendron in Malesia
. P. Noordoff Limited, Groningen,
The Netherlands.
23. Sleumer, H.O. 1980. Past and present taxonomic systems of
Rhododendron
based on
macro-morphological characters. In. J.L. Luteyn [ed.].
Contributions Toward a Classification of
Rhododendron
. 1926. The New York Botanical Garden, Bronx, New York.
24. Vogelmann, T.C., J.N. Nishio, and W. K. Smith. 1996. Leaves and light capture: Light
propagation and gradients of carbon dioxide fixation within leaves.
Trends in Plant
Science
1: 65-70.
