JARS v57n2 - Use of an Ericoid Mycorrhizal Fungus to Improve Rooting and Acclimation of Difficult-to-root Cultivars of Rhododendron
Use of an Ericoid Mycorrhizal Fungus to Improve Rooting
and Acclimation of Difficult-to-root Cultivars of Rhododendron
Mark C. Starrett, David A. Heleba and Adam R. Wheeler
University of Vermont
Burlington, Vermont
Summary
Inoculation of microshoots of Rhododendron 'Elviira'*, R. 'Ginny Gee' and R. 'Nova Zembla' with an isolate of the ericoid mycorrhizal fungus Hymenoscyphus ericae (Read) Korf and Kernan did not improve initial rooting or survival after 1 month incubation in a growth chamber when compared to non-inoculated microshoots. Additionally, no survival or shoot growth benefits were apparent from mycorrhizal inoculation of plantlets after 3 months in a greenhouse. Although the cultivar selections, R. 'Elviira' and R. 'Ginny Gee' are purportedly difficult to root and establish, >90% of all plantlets survived and acclimatized to greenhouse conditions regardless of treatment. Inoculated plantlets of Rhododendron 'Ginny Gee' had significantly less colonization (17%) when compared with R. 'Elviira' (49%) and R. 'Nova Zembla' (41%). However, there was no correlation between shoot growth response and extent of root colonization.
Introduction
The term "mycorrhizae" literally means "fungus-root" and was a term attributed to the German botanist Albert Frank in 1885 (Bagyaraj, 1986). Mycorrhizae are fungi that form symbiotic associations with the root systems of most plants found on land. Surprising to most is the fact that mycorrhizas and not roots directly are a primary mechanism of nutrient uptake by many land plants (Smith and Read, 1997). Mycorrhizae are said to "colonize" the roots of plants as opposed to "infect" the roots, as this latter term has negative connotations. Mycorrhizal fungi, in general, aid in water acquisition as well as mineral nutrient uptake (mostly phosphorus), and in exchange the host plant provides the fungus with simple sugars that are byproducts of the process of photosynthesis.
There are several different classifications of mycorrhizae which are based on their morphological characteristics and on the species of plants that they form associations with. The most common are the Vesicular-Arbuscular (VA) mycorrhizae which are found in the roots of magnolias, willows, crabapples and apples, and many garden flowers (Smith and Read, 1997). VA mycorrhizae are mostly contained within the root cells of the host plant with only fine strands (hyphae) of the fungus extending out into the surrounding soil. VA mycorrhizae generally require a host plant to survive and multiply, thus their pure culture and production in the laboratory has been limited. However, the majority of the commercially available mycorrhizal products on the market today contain VA mycorrhizae.
Ectomycorrhizae are the next most prevalent mycorrhizal fungi. These are found in association with beech, birch, oaks, firs, hemlock, pines and spruce. Truffles, an edible fungal delicacy, are the spore-producing structures of a type of ectomycorrhizal fungus found in association with beech and oak trees. The difference between the ectomycorrhizae and the VA mycorrhizae is that the ectomycorrhizae form a web-like sheath around the outside of the roots of the host plant and generally the fungus does not penetrate the cells of the root. This sheath can help absorb nutrients from the surrounding soil, exchange nutrients from one neighboring plant to another, as well as help protect the roots of the host plant from attack by pathogenic fungi.
Another group of mycorrhizal fungi are more specialized and host specific. These mycorrhizae are found only in the order of plants known as the "Ericales". This order of plants includes the families Empetraceae ("crowberry"), Epicridaceae ("epicrid"), Ericaceae ("heath") and Monotropaceae ("Indian pipe"), as well as others. Within this order of plants, there are three types of mycorrhizae: arbutoid, monotropoid, and ericoid. Arbutoid mycorrhizas specifically colonize plants in the genera Arbutus ("madrone") and Arctostaphyllos ("bearberry") and have structures similar to ectomycorrhizae. Monotropoid mycorrhizae also have similarities to ectomycorrhizae; however, these fungi only form associations with plants in the genus Monotropa ("Indian pipe"). Indian pipes are achlorophyllus, meaning they are plants which lack chlorophyll and therefore do not obtain their energy from sunlight. Indian pipes are highly dependent on mycorrhizal fungi to channel sugars to them from other plants in their surrounding environment so that they can survive and carry out their life cycle.
The ericoid mycorrhizal are also highly specialized mycorrhizae within the order Ericales . These mycorrhizal fungi only colonize roots of plants in the families Empetraceae , Epicridaceae and Ericaceae . Several fungi have been noted to colonize the roots of plants in these families and these include: Claveria (Peterson et al., 1980), Hymenoscyphus (Read, 1974), Oidiodendron (Couture et al., 1983; Currah et al., 1993; Dalpé, 1986; Dalpé, 1991; Douglas et al., 1989; Pearson and Read, 1973; Xiao and Berch, 1992) and Scytalidium (Dalpé et al., 1989). The most commonly investigated ericoid mycorrhizal fungus is Hymenoscyphus ericae (Read) Korf and Kernan [syn. Pezizella ericae Read]. While the other fungi have been shown to colonize the roots of the host, none except for H. ericae , have been shown to result in any benefits to the host. Without confirmation of a mutualistic symbiosis, one cannot classify the relationship as "mycorrhizal."
Hymenoscyphus ericae colonizes the cortical cells of roots of the host and also forms a loose "weft" of fungal hyphae around the root. The primary function of this fungus appears to be in the uptake of water as well as phosphorus and nitrogen (Smith and Read, 1997). The fungus colonizes the fine "hair roots" of the host and provides hyphal extensions beyond the root to obtain the mineral nutrients required by the plant. Unlike most plants, ericaceous plants lack "root hairs" which generally function in the uptake of water and nutrients. In the case of ericaceous plants, the fine "hair roots" colonized with ericoid mycorrhizae function in a manner similar to "root hairs" found in other plant families.
Hymenoscyphus ericae is a widespread ericoid mycorrhizal fungus found on North American and European continents (Moore-Parkhurst and Englander 1981; Read, 1983). Hymenoscyphus ericae has demonstrated mycorrhizal associations with Calluna vulgaris (L.) Hull [heather, (Read, 1974)], Rhododendron chapmanii Gray [Chapman's rhododendron, now classified as R. minus var. chapmanii , (Barnes and Johnson, 1986)], Rhododendron maximum L. [rosebay rhododendron, (Moore-Parkhurst and Englander, 1981)], Rhododendron ponticum L. (Duddridge and Read, 1982), Vaccinium angustifolium Ait. [lowbush blueberry, (Couture et al., 1983)], and V. corymbosum L. [highbush blueberry, (Lareau, 1985)].
Similar to the widespread distribution of ericoid mycorrhizae is the widespread distribution of host species in the heath family, Ericaceae . Plants in this family are indigenous to almost every region of the world. These plants often thrive on otherwise unproductive sites because they develop specialized mycorrhizal associations common to most, if not all, ericaceous plants (Jackson and Mason, 1984). Many ericaceous plants are found on acidic, nutrient-poor soils high in organic matter. Association with an ericoid mycorrhizal fungus is often necessary for survival in such conditions.
Micro-propagation of Rhododendron spp. L. (rhododendron) has become a popular method of propagating these ericaceous species (Chée, 1985). Micro-propagation is generally performed under aseptic conditions. Therefore, the resultant plants lack the "normal" mycorrhizal association they generally develop when produced by seed in nature. Micro-propagated plants lacking ericoid mycorrhizae may not perform as well as those which have been appropriately colonized either during initial growth in the greenhouse/nursery or when planted out in the landscape. An inoculum consisting of an ericoid mycorrhizal fungus combined with a soilless, peat-based medium that can be used in conjunction with micro-propagation protocols may benefit the nursery industry during production of ericaceous plants.
A micro-propagation protocol developed by Anderson (1978) is used currently for rapid, commercial propagation of rhododendron. For this protocol, a soilless, peat-based medium may be used for direct rooting following production of microshoots or microshoots may be induced to root in the agar medium in vitro (Anderson, 1978). Micro-propagated rhododendrons are often rooted ex vitro in a peat/vermiculite medium (Barnes and Johnson, 1986; Smagula and Litten, 1989). Often, the greatest losses in micro-propagation occur during plantlet acclimatization to greenhouse conditions (Preece and Sutter, 1991). Typically, 10% of micro-propagated plants in the Ericaceae either die or do not attain market standards during acclimatization, resulting in significant commercial losses (Lemoine et al., 1992). Losses of 20% to 40% have been reported for plantlets of Rhododendron during acclimatization (Anderson, 1978). Unfortunately, ericaceous plants have exhibited mixed responses when inoculated with ericoid mycorrhizal fungi during micro-propagation (Barnes and Johnson, 1986; Berta and Gianinazzi-Pearson, 1986; Lareau, 1985; Smagula and Litten, 1989; Starrett et al., 1995; Starrett et al., 2001a; Starrett et al., 2002b). Therefore, the primary objective of the following research was to determine the effects of ericoid mycorrhizae on ex vitro acclimatization and subsequent growth of micro-propagated plants of Rhododendron 'Elviira', R. 'Ginny Gee', and R. 'Nova Zembla'. Rhododendron 'Elviira' and R. 'Ginny Gee' were selected because they are generally considered "difficult-to-propagate," while R. 'Nova Zembla' was chosen for its noted ease of propagation.
Rhododendron 'Elviira' is a relatively new introduction from the University of Helsinki, Finland (1986). This cultivar is being planted for its showy red flowers in late May and exceptional bud hardiness [-34°C (-29°F)]. Rhododendron 'Elviira' also has a compact and low-spreading habit of growth. This cultivar is a hybrid of R. brachycarpum ssp. brachycarpum Tigerstedtii Group x R. forrestii .
Rhododendron 'Ginny Gee' is an older hybrid from 1979. This cultivar is extremely floriferous and is covered with pink and white flowers in June. This is a hybrid of R. keiskei 'Yaku Fairy' x R. racemosum and has received the highest awards of the ARS. Rhododendron 'Ginny Gee' has a dense and low habit of growth.
Rhododendron 'Nova Zembla' is a long-time industry standard and has been since its introduction in 1902. This cultivar is a hybrid of R. 'Parson's Grandiflorum' and an unknown red flowered hybrid. Rhododendron 'Nova Zembla' is floriferous and cold-hardy to -32°C (-25°F). Flowers are a raspberry red color with a darker blotch and are produced in early June.
Trials were conducted to determine if addition of the ericoid mycorrhizal fungus Hymenoscyphus ericae would improve rooting, acclimation and survival, and initial growth of select cultivars of rhododendron.

|
Materials and Methods
PREPARATION OF FUNGAL INOCULUM
The ericoid mycorrhizal fungus utilized in this research was Hymenoscyphus ericae (#32985) from the American Type Culture Collection (Rockville, MD). This strain was originally isolated from roots of Calluna vulgaris L. growing in England by Dr. David Read (Read, 1974). Despite the fact that it was originally in association with heather, this strain has been able to regularly colonize root cells of other ericaceous species (Gorman, 2000; Starrett, 2001a; Starrett 2001b). For this reason, this strain was selected for use in this study. Pure cultures of H. ericae were grown out on malt agar Baccto® malt agar (45 g•L -1 ) for thirty days. Unlike VA mycorrhizae, H. ericae does not require a host plant to survive and grows slowly but easily on a nutrient medium. Fungal cultures were placed in a controlled environment chamber maintained at a constant temperature of 24°C (75°F) in the dark.
After 1 month of growth on malt agar, 5 mm 3 of fungal hyphae were aseptically removed and transferred to each of 50 baby food jars containing 150 ml (approx. 2/3 cup) of Scotts ® MetroMix ® 350 (contains: peat, vermiculite, starter nutrients, wetting agents and processed bark with a pH of 5.0 – 6.5). Prior to inoculation with the fungus, the MetroMix medium was added to each baby food jar and moistened with 20 ml (4 teaspoons) of a 0.5% sucrose (sugar) solution. The sugar is a necessary energy source for the fungus to sustain growth through the mix. The baby food jar was capped with a Magenta ® B-cap and all jars were autoclaved at 121°C (250°F) for 15 minutes. After the fungal "plug" was inserted into the autoclaved mix, an additional 5 ml (1 teaspoon) of the sugar water solution was applied to the fungal plug to firm the fungus into the mix. These fungal cultures were placed back in a controlled-environment chamber maintained at a constant temperature of 24°C (75°F) in the dark and grown on for another 30 days.
PREPARATION OF RHODODENDRON MICROSHOOTS
Microshoots of Rhododendron 'Elviira', 'Ginny Gee' and 'Nova Zembla' were obtained from Brigg's Nursery (Olympia, WA). Fifty microshoots, each approximately 3.5 cm (1.4 in.) in length, were selected and placed vertically to a depth of 1 cm (0.4 in.) in Magenta ® GA-7 vessels. Each vessel contained 75 ml (1/3 cup) of 1/4 strength Woody Plant Medium [(WPM) Lloyd and McCown, 1980], solidified with 0.8% TC agar and supplemented with 0.025 g•L -1 myoinositol, 0.6 g•L -1 activated charcoal, 30 g•L -1 sucrose and 5 mg•L -1 of the potassium (K) salt of 1H-indole-3-butyric acid (K-IBA). The pH of the resultant medium was adjusted to 5.2 prior to autoclaving. Magenta vessels were capped with Magenta B-caps and sealed with Parafilm ® "M". Cultures were placed in a controlled-environment chamber maintained at 24+1°C (75+2°F) with a 16 hour photoperiod provided by six cool-white fluorescent tubes and two 25-watt, soft-white incandescent bulbs suspended 24 cm (9.5 in.) above the tops of the Magenta vessels. Tubes and bulbs provided a photosynthetic photon flux [PPF (400-700 nm)] of approximately 144 μmol•m -2 •sec -1 as measured at the tops of the vessels. Microshoots were incubated in the root induction medium for 14 days at which time root initials were visible.
One hundred microshoots with root initials present were selected for uniformity in size from each cultivar. Fifty of each cultivar were transferred to one of two 400-cell plug trays [cell vol. = 3.5 ml (0.2 in.3); cell depth = 2.5 cm (1 in.)] containing either autoclaved 1:1 (vol.:vol.) perlite:autoclaved MetroMix 350 with 1 mo. old cultures of H. ericae , and the remaining fifty microshoots of each cultivar were transferred to a tray containing the same medium which lacked the inoculum ( H. ericae ). The non-inoculated medium contained the identical autoclaved MetroMix moistened with the sucrose solution as the medium which contained the mycorrhizal fungus. Non-inoculated microshoots were handled first to avoid the risk of contamination by mycorrhizae from inoculated microshoots. Microshoots in all flats were inserted vertically into the medium to a depth of 1 cm (0.4 in.) and were moistened with distilled water until excess water was draining from the medium. The flats were then covered with a clear plastic propagation dome to help retain humidity. The trays containing inoculated vs. non-inoculated media were spatially separated within the chamber to avoid the risk of contamination of the non-inoculated tray. Flats were placed on an electric mat which provided bottom heat maintained at 24+1°C (75+2°F). Air temperature, as measured within the dome over the flat, was maintained at 25+1°C (77+2°F) during the day and 22+1°C (72+2°F) at night. A daylength of 16 hours was provided with an average photosynthetic photon flux [PPF (400-700nm)] of 140 μmol•m -2 •sec -1 as measured inside the propagation dome. An ultrasonic humidifier was utilized to help maintain humidity within the chamber. Microshoots were incubated at approximately the following relative humidity levels: week #1 = 100%, week #2 = 90%, week #3 = 80% and week #4 = 70%.
At the end of 1 month, microshoots were assessed for survival and rooting. Each microshoot was carefully extracted from the plug tray using tweezers. Rootballs were retained intact to assure a better transition to greenhouse conditions. The surface of the rootballs were examined for the presence or absence of visible roots.
Microshoots were then inserted in plastic 6-cell packs containing a 1:1 (vol.:vol.) autoclaved Fafard ® 3B potting mix (containing peat moss, perlite, vermiculite and processed bark, with a pH of 5.5 – 6.5) and autoclaved MetroMix 350 previously inoculated H. ericae or to the same medium lacking mycorrhizal inoculum.
Following transplant, all cell packs were then transferred to a greenhouse and watered with deionized water to leach out any remaining sucrose solution from the potting medium. To continue to prevent the chance of cross contamination of the non-inoculated plants by media from the inoculated plants, a Plexiglas⢠shield surrounded each six-pack container.
Natural light was supplemented by sodium-vapor high intensity discharge (HID) lamps which were suspended 1.4 m (4.5 ft.) above the cell packs. Light reaching the plants in the greenhouse ranged from a maximum PPF of 190 – 220 μmol•m-2•sec-1 during the day and 30 – 40 μmol•m-2•sec-1 during the transition to night. HID lights were illuminated for 16 hours each day. Greenhouse temperatures were maintained in an air-conditioned greenhouse at 25+2°C (77+4°F) during the day and 20+2°C (68+4°F) at night. Plants were watered daily with deionized water and fertilized weekly with 1/8 strength Woody Plant Medium [(nutrient salts only) Lloyd and McCown, 1980]. Plants were grown in the greenhouse for a period of 3 months and then harvested (Fig. 1).

|

|

|
||
|
Figure 1. (L. to R.) Plantlets of Rhododendron 'Elviira'*, 'Ginny Gee' and 'Nova Zembla' 3 months
after placement in the greenhouse.
Photo by the authors |
||||
To determine whether the presence of H. ericae influenced initial growth of the micro-propagated rhododendrons, the following were measured: shoot number, shoot length, leaf number, leaf area and shoot dry weight. Samples were dried in a forced air drying oven at 55°C (131°F) for 4 days.
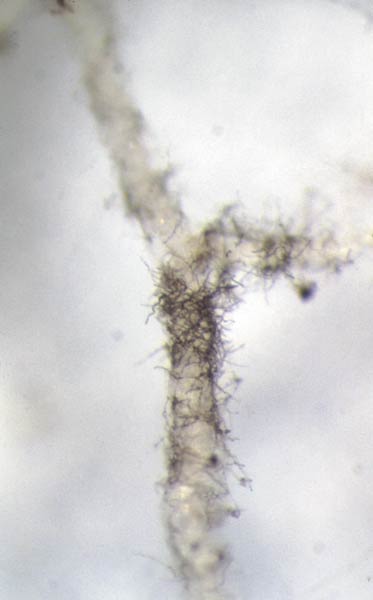
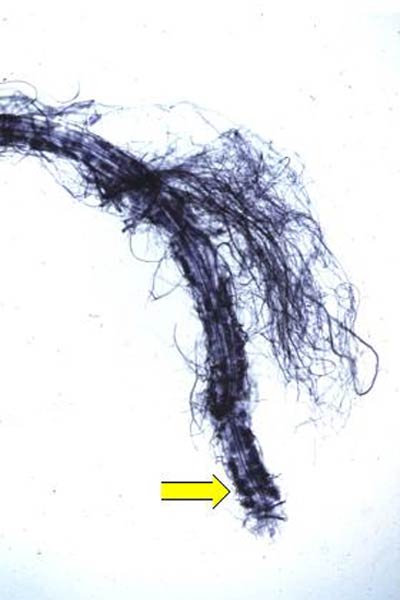
To determine if inoculated plants were colonized by H. ericae , roots were washed free of the growing medium and a uniform portion of each root system, weighing approximately 100 mg [(0.0004 oz) fresh weight], was selected from every plant. Roots were taken proximal to the microshoot and included a primary root with associated secondary roots. The roots were then cleared and stained following a protocol adapted from Brundrett et al. (1984) and examined using bright field microscopy (Brundrett et al., 1984). The stain, chlorazol black E, was used which is a fungal-specific stain and only adheres to the fungus, if present (Fig. 2). The root system was spread out evenly over the surface of a glass microscope slide and was covered with a 22 mm x 40 mm (0.9 in. x 1.6 in.) glass cover slip. The slide was divided into a 5 x 10 square grid resulting in 50 squares. One grid square was equal to 2% of the root system. If a root cell was colonized within a grid square, then that portion of the root system was classified as, "colonized". The number of squares colonized was totaled to determine the extent of root colonization by the mycorrhizal fungus.
|
|
|
|
Figure 2. (Left) Rhododendron root covered in hyphae of
Hymenoscyphus ericae
.
(Right) Rhododendron root cleared and stained with chlorazol black E. Yellow arrow indicates cells colonized by H. ericae . Photo by the authors |
||
The experimental design was a randomized complete block design with treatments in a 2 x 3 factorial structure. There were 2 media types (+/- H. ericae ) and 3 rhododendron cultivars (R. 'Elviira', R. 'Ginny Gee' and R. 'Nova Zembla'). There were 6 six-packs per treatment per replication with 6 plantlets per six pack. The experiment was repeated 5 times with 30 days between replication for manageability of harvest and data collection.
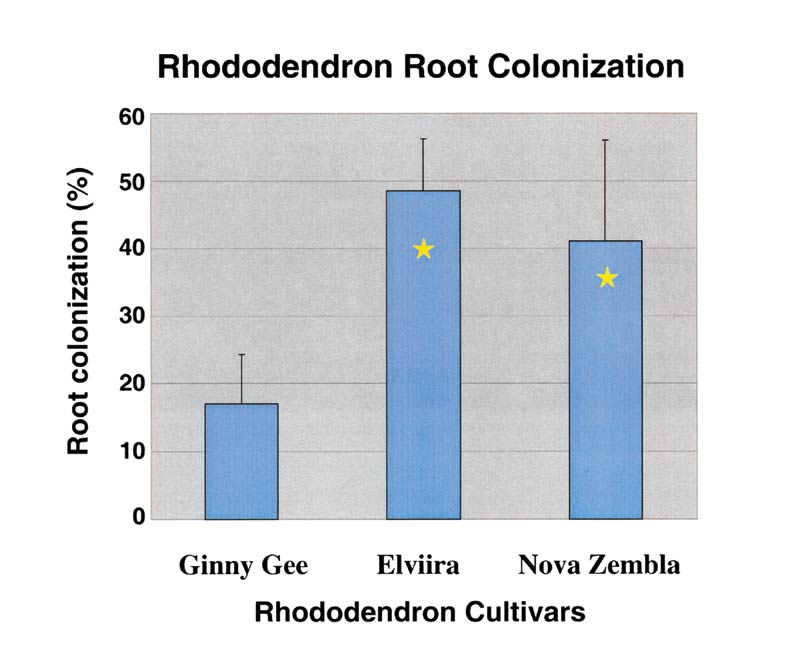
Statistical analyses were performed using SAS (SAS Institute, 1989a: SAS Institute, 1989b). Comparisons of colonization intensity and all shoot growth measures are based on analysis of variance (ANOVA) and general linear modeling (GLM) in SAS. In order to satisfy the homogeneity of variance assumption associated with the analysis of variance, data corresponding to percent colonization was arcsine square root transformed. Though statistical significance was based on transformed data for percent colonization, means presented in the figure represent untransformed data (Fig. 3). Pairwise comparisons are based on Fisher's LSD (p <0.05). PROC CORR (Pearson's Correlation) was used to determine if extent of root colonization was correlated with any measured shoot response.
|
| Figure 3. Rhododendron root colonization. |
Results
All of the inoculated plantlets became colonized by H. ericae . All the non-inoculated plantlets were not colonized. Inoculated plantlets of Rhododendron 'Ginny Gee' had significantly less colonization (17%) when compared with R. 'Elviira' (49%) and R. 'Nova Zembla' (41%) (Fig. 3). However, there was no correlation between extent of root colonization and any of the shoot measurements taken. There were no significant differences noted in plant survival between inoculated and non-inoculated plantlets either in the growth chamber or after acclimating to greenhouse conditions. Greater than 90% of all plantlets survived and acclimatized to greenhouse conditions regardless of treatment.
Additionally, there were no significant differences between inoculated and non-inoculated plantlets in any of the shoot measurements taken: shoot length, shoot number, leaf number, leaf area, and shoot dry weight.
Discussion
There were no apparent benefits from inoculation with H. ericae in establishment or initial growth of the three cultivars of rhododendron selected when compared to non-inoculated plants. While some studies have demonstrated an increase in shoot growth of ericaceous plants when grown in vitro [aseptic conditions (Gorman, 2000; Starrett, et al., 2002b)], others have demonstrated that once the plants are placed in a growth chamber or greenhouse, these growth benefits are not sustained (Barnes and Johnson, 1986; Starrett et al., 2002b). The lack of response in those plants colonized by H. ericae may be attributed to the fact that all plants in this study were supplied with sufficient mineral nutrients in the 1/8-strength Woody Plant Medium. Woody Plant Medium used without dilution is the industry standard for proper growth and development of ericaceous plants during micro-propagation. Providing the rhododendron plantlets with a very dilute nutrient solution on a weekly basis should not have masked the effects of inoculation with and colonization by H. ericae based on previous studies (Starrett, data not presented). Studies have shown that if adequate nutrients are present, the benefits of mycorrhizae may not be evident (Bannister and Norton, 1974; Goulart et al., 1993; Read, 1996; Smagula and Litten, 1989; Stribley, et al., 1975; Stribley and Read, 1976). Hymenoscyphus ericae has been shown to be most advantageous to plants growing in less than ideal conditions, especially in nutrient-poor soils (Stribley and Read, 1976).
Despite the fact that R. 'Elviira' and R. 'Ginny Gee' are purportedly difficult to propagate, greater than 90% of all microshoots rooted and were acclimatized to greenhouse conditions regardless of the presence or absence of the ericoid mycorrhizal fungus, H. ericae . Therefore, the protocol used in this research for acclimation and establishment of these rhododendron cultivars should be considered as a potential method to establish them when grown from a micro-propagated source.
Acknowledgements
Funding for this research was provided by the American Rhododendron Society. Microshoots of all rhododendron cultivars used in this study were supplied by Lynne Caton, propagation manager, Briggs Nursery, Inc., Olympia, WA.
Literature Cited
Anderson, W.C. 1978. Rooting of tissue cultured rhododendrons.
Proc. Intl. Plant Prop. Soc.
28:135-139.
Bagyaraj, D.J. 1986. Mycorrhizal associations in crop plants and their utilization in agriculture, p. 59- 84. In:
M.C. Nair and S. Balakrishnan (eds.).
Beneficial Fungi and their Utilization
. Scientific Publishers, Jodhpur,
India.
Bannister, P. and W.M. Norton. 1974. The response of mycorrhizal and nonmycorrhizal rooted cuttings of heather
(
Calluna vulgaris
(L.) Hull) to variations in nutrient and water regimes.
New Phytol.
73:81-89.
Barnes, L.R. and C.R. Johnson. 1986. Evaluation of ericoid mycorrhizae and media on establishment of micropropagated
Rhododendron chapmanii
, Gray.
J. Environ. Hort.
4:109-111.
Berta, G. and V. Gianinazzi-Pearson. 1986. Influence of mycorrhizal infection on root development in
Calluna vulgaris
(L.) Hull seedlings, p. 673-676. In: V. Gianinazzi-Pearson and S. Gianinazzi (eds.). Physiological and
genetical aspects of mycorrhizae. Proc. 1st European Symp. on Mycorrhizae, Dijon, France, 1-5 July 1985.
Brundrett, M.C., Y. Piché, and R.L. Peterson. 1984. A new method for observing the morphology of vesiculararbuscular
mycorrhizae.
Can. J. Bot.
62:2128-2134.
Chée, R. 1985. The history of rhododendron micropropagation provides some tips for success.
Amer. Nurseryman
161(10):42-47.
Couture, M., J.A. Fortin, and Y. Dalpé. 1983.
Oidiodendron griseum
Robak: An endophyte of ericoid mycorrhiza
in Vaccinium spp.
New Phytol.
95:375-380.
Currah, R. S., A. Tsuneda, and S. Murakami. 1993. Conidiogenesis in
Oidiodendron periconioides
and ultrastructure of
ericoid mycorrhizas formed with
Rhododendron brachycarpum
.
Can. J. Bot.
71:1481-1485.
Dalpé, Y. 1986. Axenic synthesis of ericoid mycorrhiza in
Vaccinium angustifolium
by
Oidiodendron
species.
New Phytol.
103:391-396.
Dalpé, Y. 1991. Endomycorrhizal status of the genus
Oidiodendron
.
Can. J. Bot.
69:1712-1714.
Dalpé, Y., W. Litten and L. Sigler. 1989.
Scytalidium vaccinii
a new species, an ericoid endophyte of
Vaccinium
angustifolium
roots.
Mycotaxon
35:371-378.
Douglas, G.C., M.C. Heslin, and C. Reid. 1989. Isolation of
Oidiodendron maius
from
Rhododendron
and ultra-structural
characterization of synthesized mycorrhizas.
Can. J. Bot.
67:2206-2212.
Duddridge, J. and D.J. Read. 1982. An ultrastructural analysis of the development of mycorrhizas in
Rhododendron
ponticum
.
Can. J. Bot.
60:2345-2356.
Gorman, N.R. 2000. Use of ericoid mycorrhizal fungi to reduce inputs in commercial production of ericaceous plants.
Master's Thesis. Dept. of Plant and Soil Science, University of Vermont, Burlington.
Goulart, B.L., M.L. Schroeder, R.L. Darnell, J.R. Clark and W.F. Wilcox. 1993. Blueberry mycorrhizae: current
knowledge and future directions. In: Proc. Fifth Intl. Symp.
Vaccinium
Culture. Acta Hort. 346:230-239.
Jackson, R.M. and P.A. Mason. 1984. Mycorrhiza. Studies in biology. No. 159. Edward Arnold, London.
Lareau, M.J. 1985. Rooting and establishment of in vitro blueberry plantlets in the presence of mycorrhizal
fungi, p. 197-201. In: K. Pliszka (ed.). Third Intl. Symp.
Vaccinium
Culture, Warsaw, Poland, 24-28 July 1984.
Acta Hort.
vol. 165.
Lemoine, M.C., S. Gianinazzi, and V. Gianinazzi-Pearson. 1992. Application of endomycorrhizae to commercial production
of
Rhododendron
microplantlets.
Agronomie
12:881-885.
Lloyd, G. and B. McCown. 1980. Commercially-feasible micropropagation of mountain laurel,
Kalmia latifolia
, by use
of shoot-tip culture.
Proc. Intl. Plant Prop. Soc.
30:421–437.
Moore-Parkhurst, S. and L. Englander. 1981. A method for the synthesis of a mycorrhizal association between
Pezizella
ericae
and
Rhododendron maximum
.
Mycologia
73:994-997.
Pearson, V. and D.J. Read. 1973. The biology of mycorrhiza in the Ericaceae. I. The isolation of the endophyte and
synthesis of mycorrhizas in aseptic culture.
New Phytol.
72:371-379.
Peterson, T.A., W.C. Mueller and L. Englander. 1980. Anatomy and ultrastructure of a
Rhododendron
root-fungus
association.
Can. J. Bot.
58:2421-2433.
Preece, J.E. and E.G. Sutter. 1991. Acclimatization of micropropagated plants to the greenhouse and field, p. 71-93.
In: P.C. Debergh and R.H. Zimmerman (eds.).
Micropropagation
. Kluwer Academic Publ., Boston.
Read, D.J. 1974.
Pezizella ericae
sp. nov. the perfect state of a typical mycorrhizal endophyte of Ericaceae.
Trans. Brit. Mycol. Soc.
63:381-419.
Read, D.J. 1983. The biology of mycorrhiza in the Ericales.
Can. J. Bot.
61:985-1004.
Read, D.J. 1996. The structure and function of the ericoid mycorrhizal root.
Ann. Bot.
77:365-374.
SAS Institute Inc. 1989a. SAS/STAT
®
user's guide, version 6, fourth ed. vol. 1. SAS Inst., Inc. Cary, N.C.
SAS Institute Inc. 1989b. SAS/STAT
®
user's guide, version 6, fourth ed. vol. 2. SAS Inst., Inc. Cary, N.C.
Smagula, J.M. and W. Litten. 1989. Effect of ericoid mycorrhizal isolates on growth and development of lowbush blueberry
tissue culture plantlets.
Acta Hort.
241:110-114.
Smith, S.E. and D.J. Read. 1997.
Mycorrhizal Symbiosis
. 2nd ed. Academic Press. Boston.
Starrett, M.C., F.A. Blazich, L.F. Grand, and S.R. Shafer. 1995. Response of seedlings of highbush blueberry to in
vitro ericoid mycorrhizal innoculation [sic.]. Proc. Southern Nurserymen's Assoc. Res. Conf., 40th Annu. Rpt. p. 266-268.
Starrett, M.C., F.A. Blazich, L.F. Grand, and S.R. Shafer. 2001a. In vitro colonization of micropropagated
Pieris
floribunda
by ericoid mycorrhizae. I. Establishment of mycorrhizae on microshoots.
HortScience
. 36:353-356.
Starrett, M.C., F.A. Blazich, L.F. Grand, and S.R. Shafer. 2001b. In vitro colonization of micropropagated
Pieris
floribunda
by ericoid mycorrhizae. II. Effects on acclimatization and growth.
HortScience
. 36:357-359.
Stribley, D.P. and D.J. Read. 1976. The biology of mycorrhiza in the Ericaceae. VI. The effects of mycorrhizal infection
and concentration of ammonium nitrogen on growth of cranberry (
Vaccinium macrocarpon
Ait.) in sand culture.
New Phytologist
. 77:63-72.
Stribley, D.P., D.J. Read and R. Hunt. 1975. The biology of mycorrhizae in the Ericaceae V: The effects of mycorrhizal
infection, soil type and partial soil sterilization (by gamma irradiation) on growth of cranberry (
Vaccinium
macrocarpon
Ait.).
New Phytol
. 75:119-130.
Xiao, G. and S.M. Berch. 1992. Ericoid mycorrhizal fungi of
Gaultheria shallon
.
Mycologia
84:470-471.
Authors
Mark C. Starrett1, Assistant Professor of Horticulture, Univ. of Vermont, David A. Heleba, Research Technician, Adam R.
Wheeler, Graduate Assistant
* Name is not registered.
