JARS v57n3 - Companion Plants: Rhododendrons and Ferns Belong Together
Companion Plants: Rhododendrons and Ferns Belong Together
Dr. Robert Means
Winston Salem, North Carolina
Ferns with Rhododendrons
Rhododendrons and ferns naturally grow together and have done so for millions of years. Imprints of their existence on chunks of coal date from the Carbonaceous age. Both are social plants growing in colonies, and natural cultivated gardens should include them whether on a standard building lot or a more generous woodland. The preferred habitat of ferns is the transition zone of subdued light between the darkness of the deep woods and the sunny meadows, though many genera and species have adjusted to the extremes. On rock strewn stream banks where absence of trees permits light to penetrate the forest canopy they are at their best. Here, acidic compost, mosses, and liverworts conserve moisture in times of drought to constantly nourish thirsty roots. Here and there decaying lichen-covered tree remnants are disappearing into the thick layer of plant debris of the forest floor. Scattered moss-covered stone outcrops add to the primitive scene. Space is shared with Kalmia , Pieris and deciduous azaleas. It is crowded yet silent except for the sound of moving water and now and then a winged inhabitant. It is a refreshing place for happy thoughts and memories.
Attempting to recreate this lush environment would be an exciting challenge for the gardener. It should not appear too wild and unkempt or neighbors might complain. It should be controlled and modified to aesthetically blend in and harmonize with existing surroundings. Unobtrusive utilitarian pathways may be paved with mulch, gravel, brick, grass, moss, or stone. Thinning, pruning, dividing, moving, and insect control are always ongoing tasks.
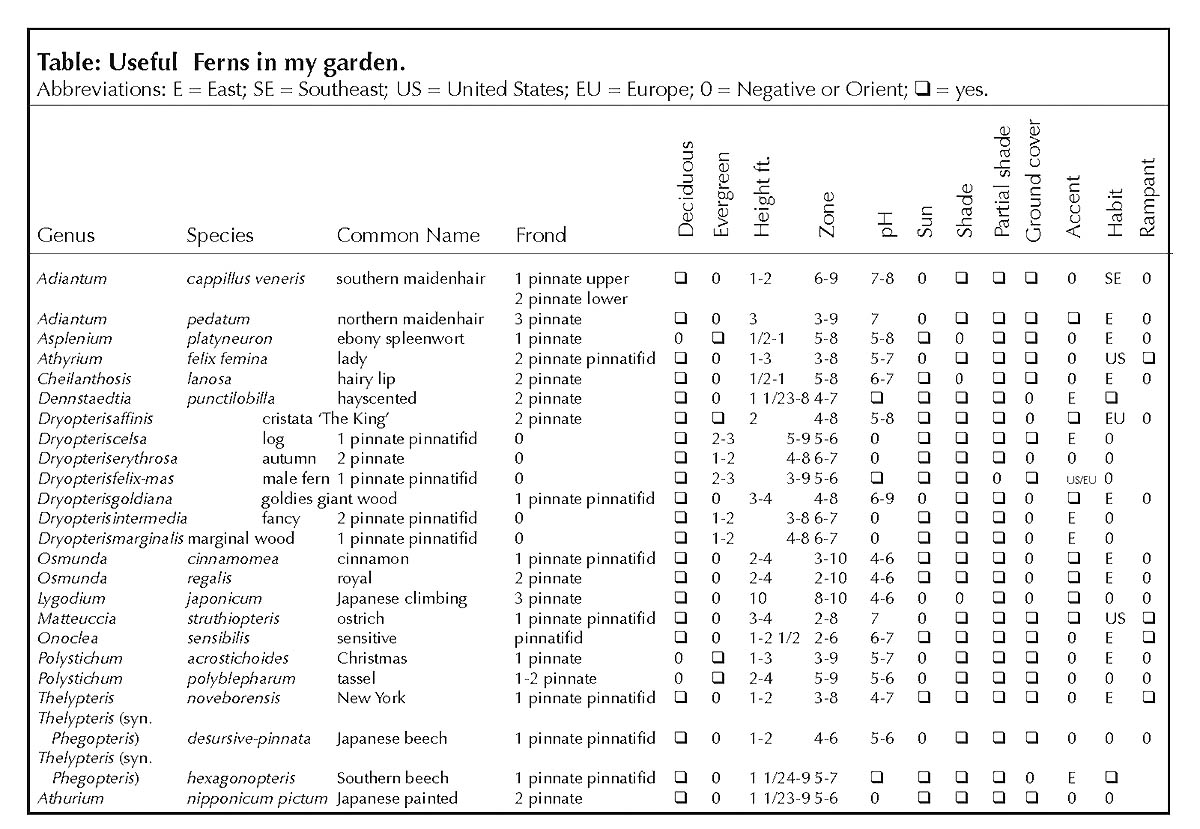
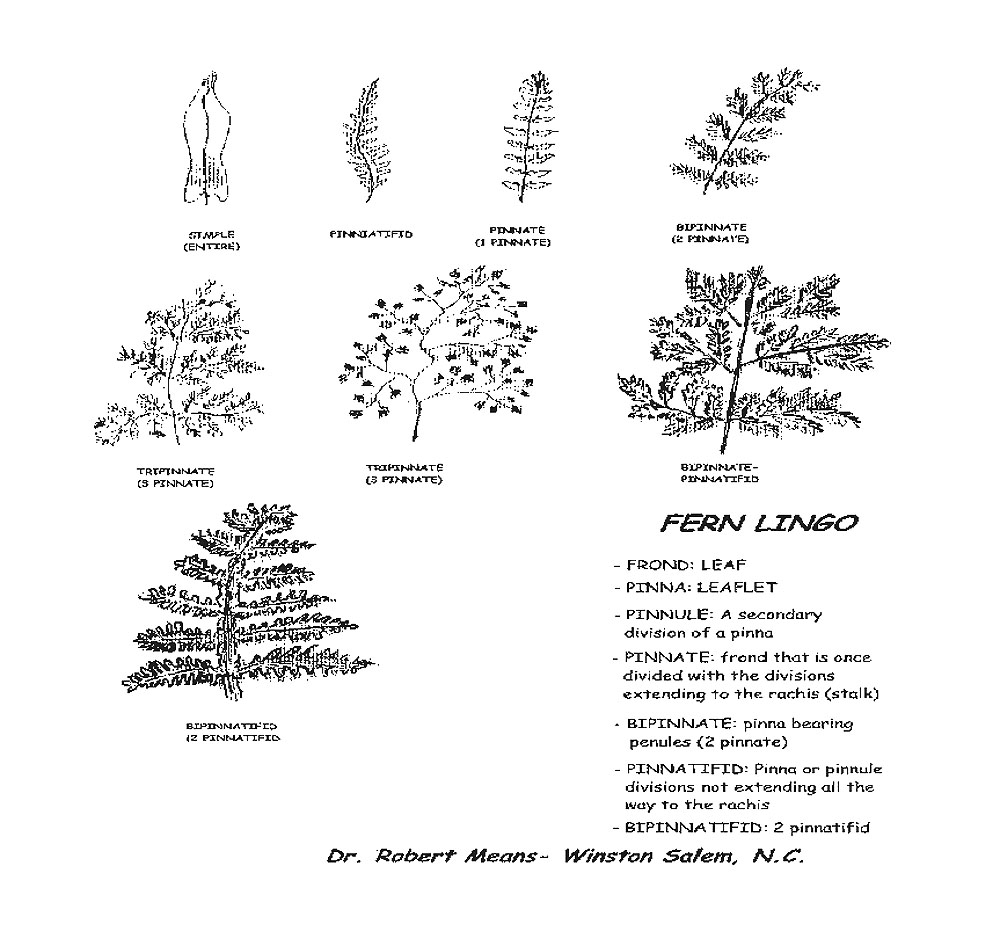
Ferns and rhododendrons, once established, require little care. Ferns, especially, despite their apparent delicacy, have little difficulty with predators and disease. Some like the Southern beech fern and the sensitive fern are so happy and carefree that they will overtake their neighbors. These rampant ones, though beautiful, should be planted only where their boundaries can be controlled as between a foundation and concrete walk or driveway. Some, like the Southern maidenhair fern and the ebony spleenwort, have adapted to the more sunny positions with alkaline soil and are often planted among limestones sometimes called cobbles. Others, like the statuesque cinnamon fern or Goldie's fern, may be used as specimen or accent plants between and among other plants. The low and medium sized growers may be used in drifts or mass plantings in front of or beneath lanky growing plants. They are used to provide conformity, form, stability, varying textures and tints of green, silver, and bronze. Bulbs, hostas, Asarum, Arisaema and many other plants with similar cultural requirements may be incorporated to contribute to the seasonal interests. (See table for a list of useful ferns in my garden. See below for frond illustrations.)
|
|---|
Growing Ferns from Spores
Propagation vegetatively is accomplished by division, buds and bulblets in some ferns, and tissue culture. The latter is used commercially to mass produce superior selections arising from sexual reproduction from spores. Growing ferns from spores is an easy, inexpensive, and fun way to add to your collection. All it requires is a little knowledge, easily obtained materials, and, most of all, patience. The Hardy Fern Foundation has proved very helpful in providing knowledge in the quarterly newsletter and plants and spores from the annual distribution. Also, spores have been obtained from my own expanding collection and from friends.
Spores are located on the underneath sides of fertile fronds. They are contained in spore cases (sporangia) which occur in clusters called sori. A sorus is covered by protective tissue flap called indusium which appears dark brown or black when the spores are ripe. This usually is from mid summer into fall. Exceptions to this are the osmundas ( Osmunda cinnamomea - cinnamon fern, O. claytoniana - interrupted fern, O. regalis - royal fern). In this genus spores form on leafless stems and are ripe when still green in early April and May and must be collected and sowed before they dry out. Another exception is the genus Polypodium (rock fern) spores which are ripe when they turn yellow.
Some recommend briefly placing the collected frond with ripe spores in a 5-10% bleach solution and then thoroughly rinsing it in running water before allowing it to dry. It is then placed between two layers of white copy paper (spore side down) and stored between the pages of an old magazine. Later after several days, or even several years when convenient, the frond may be discarded and the remaining dark powdery dust-like spores and chaff are visible. Alternately portions of fronds may be stored in envelopes.
For sowing, any commercial soilless potting medium may be used. These usually contain peat, perlite, and vermiculite. This moistened with a very dilute solution (5% recommended dilution) of liquid fertilizer with trace elements. pH may be adjusted by adding the very acid milled sphagnum for the acid loving ferns and limestone for others. The moistened medium is placed in 8-ounce clear plastic microwaveable deli dish with lid to a depth of at least 1 inch. Any excess water may be removed by compression of the medium with the back of a spoon and tilting the container. The medium must not be soggy. The lid is left on loosely and the container and contents sterilized on high in the microwave for 4-5 minutes. After cooling, the lid may be secured tightly until time to sow. Similarly, a clean plastic nursery pot may be sterilized and, after cooling, placed in a clear gallon plastic food storage bag and secured with a twist tie closure.
Contamination at the time of sowing may be minimized by removing as much chaff (spore cases and coverings) as possible. This is done in a draft-free room by tilting and gently tapping the paper so that the larger, lighter debris is moved to the edge for discard. The remaining spores may then be inverted onto the container and the spores still clinging are dispersed by sharply finger snapping. Leaving the paper in place momentarily will allow time for the dust to settle.
The containers are placed under fluorescent lights (12-24 inches) for 12-24 hours daily or in a bright east or north facing window but not in direct sunlight. When a spore germinates it forms a green shallow cup-like organism called a gametophyte (prothallus) which contains both male (antheridia) and female (archegonia) sex organs with orifices leading to the surface. When mature, and a film of water is present in the cup-like receptacles, the male gamete (sperm) is able to swim to the orifice of the female gamete (egg) of an adjoining gametophyte and cause cross-fertilization. Self-fertilization may occur but the offspring are said to be weak and often fail to grow.
The first indication that gametophytes are forming may be anywhere from one to several months after sowing. The surface of the medium becomes increasingly green and eventually, if there isn't too much overcrowding, individual cup-like gametophytes are visible. At this point twice weekly hand misting with sterile water (distilled or boiled) provides the film of water necessary for fertilization. Then, when the young new fern (sporephyte) has one or more fronds an inch or so in length, it may be moved to another container with similar medium. High humidity for at least several weeks should be maintained.
One way to transplant many small plants is to use nursery flats containing 72 small cells. Two clothes hangers are positioned on opposite sides of the flat to act as tent supports and the flat is placed in a large clear plastic leaf collection bag. Weaning from the high humidity may begin when the young sporelings are established by gradually opening the bag or making slits in it.
The most common complications that contribute to crop failure are: contamination, overcrowding, and improper medium moisture (too much or too little moisture). Despite all attempts at sterility, contaminating mosses, liverworts, blue green algae, or gray mold may appear. Early contaminants may be removed with sterile forceps or spot sprayed with a fungicide.
Gametophyte (prothali) over-crowding may be avoided by careful sowing practices and thinning with sterile forceps when necessary.
A soggy medium may be the most common cause of failure. Frequent hand misting required for fertilization may be a factor. Brief flooding of the medium for an hour or two may actually be helpful for fertilization but will doom the culture if not corrected. I do this by tilting the deli container next to a sink and siphoning off the excess water using a strip of paper towel with the long end draining into the sink. Then I may leave the lid only loosely applied for a day or two.
There are literally hundreds of hardy fern species many of which are unavailable commercially. Growing them from spores is an exciting way to discover and enjoy the unusual. Occasionally the unusual becomes more unusual when variants appear and are selected out from young sporlings. Sometimes they become quite beautiful plants when mature which adds further interest to this method of fern reproduction.
|
|---|
Dr. Means is a member of the Potomac Valley Chapter.

