JARS v61n4 - Ploidy Levels and Relative Genome Sizes of Diverse Species, Hybrids, and Cultivars of Rhododendron
Ploidy Levels and Relative Genome
Sizes of Diverse Species, Hybrids, and Cultivars of Rhododendron
Jeff R. Jones, Thomas G. Ranney, and Nathan P. Lynch
Department of Horticultural Science
Mountain Horticultural Crops Research and Extension Center
North Carolina State University, Fletcher, North Carolina
Stephen L. Krebs
David G. Leach Research Station
The Holden Arboretum, Kirtland, Ohio
Polyploidy has been an important pathway in the evolution of plants and can contribute to reproductive isolation, increased heterozygosity, novel gene combinations, modified gene expression, enzymatic multiplicity, and ultimately divergence and speciation (Soltis and Soltis, 1993; 2000; Wendel, 2000). The origins, adaptive significance, and genetic implications of polyploidy continue to be an active field of research (Bennett, 2004; Soltis et al., 2003; Chen and Ni, 2006).
For plant breeders, ploidy level is an important consideration because it can influence male and female fertility, cross fertility, plant vigor, and gene expression (Chahal and Gosal, 2002; Contreras et al., 2007; Ranney, 2006; Thomas, 1993). In some cases, polyploid plants, including rhododendrons, can have desirable characteristics including thicker leaves, enhanced vigor, and larger flowers with thicker petals that persist longer (Barlup, 2002; Hosoda et al., 1953; Kehr, 1996a; Leach, 1961). As a result, there continues to be interest in identifying naturally occurring polyploids and inducing (through mitotic doubling agents) artificial polyploids as a component of rhododendron breeding programs (Barlup, 2002; Kehr, 1996b; Paden et al., 1990; Pryor and Frazier, 1970; Leach, 1961).
Most of the more than 800 Rhododendron species have been reported to be diploid with 2n = 2x = 26. However, polyploidy occurs naturally in some rhododendron species, particularly within the Pentanthera and Rhododendron subgenera, with ploidy levels ranging from three to twelve (Ammal, 1950; Ammal et al., 1950). Sax (1930) completed one of the first surveys of chromosome numbers of rhododendron, including sixteen species, and determined a base chromosome complement of x= 13 for the genus and identified both R. calendulaceum and R. canadense (deciduous azaleas in subgenus Pentanthera ) as natural tetraploids. Nakamura (1931) surveyed fifteen Japanese species of rhododendron and found them all to be diploid. Ammal et al. (1950) completed an extensive survey of chromosome numbers and ploidy levels in 360 species of rhododendron and found the elepidote rhododendrons (subgenus Hymenanthes ), evergreen azaleas (subgenus Tsutsusi ), and the deciduous azaleas (with the exception of the tetraploid R. calendulaceum and R. canadense ) to be predominantly diploid. Ammal et al. (1950) further reported a high frequency of polyploids in the scaly-leaved species of subgenus Rhododendron , with taxa ranging from triploids to dodecaploids. In a survey of fifteen deciduous azaleas from Eastern North America, Li (1957) reported that all of the species were diploid with the exception of the tetraploid R. calendulaceum . However, a single triploid R. atlanticum was also identified among the otherwise diploid species. Among lepidotes, chromosome counts for 27 species in the tropical subgenus Rhododendron section Vireya indicated that they were uniformly diploid (Atkinson et al., 2000).
Published information on chromosome counts of specific cultivars or clones of rhododendron is less extensive. Hosada et al. (1953) completed chromosome counts on twelve cultivars of Satsuki azaleas ( R. lateritium ) and identified diploid, triploid ('Bangaku'), and tetraploid ('Banka', 'Taihei', and 'Wako') plants. Pryor and Frazier (1970) determined that the evergreen azalea hybrids 'Redwing' and 'Ablaze' were triploids and also documented the existence of mixed ploidy cytochimeras resulting from colchicine treatment. Heursel and DeRoo (1981) completed chromosome counts on 47 cultivars of evergreen azaleas and found they were all diploid with the exception of the triploid, 'Euratom'.
The chromosomes in rhododendron are small and can be difficult to view and count (Eiselein, 1994; Tolstead and Glencoe, 1991). Light microscopy is therefore not a practical method for determining ploidy levels of large numbers of individual cultivars and clones. However, flow cytometry can provide a fast and accurate determination of nuclear DNA content (genome size) that is related directly to ploidy level among closely related taxa (de Laat et al., 1987; Doležel, 1991; Doležel et al., 1998; Galbraith et al., 1983). Flow cytometry is also effective for detecting mixaploidy or cytochimeras and individual histogenic layers can be analyzed by sampling appropriate tissue (DeSchepper et al., 2001). Flow cytometry has been used successfully to determine relative DNA content and ploidy levels of Rhododendron spp. (DeSchepper et al., 2001; Eeckhaut et al., 2004; Sakai et al., 2003, 2004a, 2004b, 2006; Ureshino and Miyajima, 1998; Väinölä, 2000). De Schepper et al. (2001), for example, determined the ploidy level for six species and 88 cultivars within the evergreen azalea subgenus Tsutsusi by using flow cytometry. The vast majority were found to be diploid with the exception of three triploids ('Red Wing', 'Euratom', and 'Euratom Orange'*) and one mixaploid ('Casablanca Tetra') that was found to be diploid in the LI and LII layers and tetraploid in the LIII. Eeckhaut et al. (2004) studied various Ghent and Rustica deciduous azalea hybrids by using flow cytometry and found them to be either triploid ('Mina van Houtte', 'Daviesii', 'Quadricolor', 'Gloria Mundi', 'Van Houtte Flore Pleno', 'Norma', and 'Phébé') or tetraploid ('Nancy Waterer', 'Unique', 'Narcissiflorum', 'Jozef Baumann', 'Maja', 'Rosetta', 'Semiramis', 'Souvenir du President Carnot', 'Marie Verschaffelt', 'Batholo Lazarri'*, 'Guelder Rose', 'Coccineum Major', 'Raphael de Smet', 'General Trauff', 'Graf von Meran', 'Goldlack', 'Fénelon', and 'Racine'). In contrast to the survey by Ammal et al. (1950), Eeckhaut et al. (2004) found three clones of R. luteum to be tetraploid, not diploid. Sakai et al. (2006) identified twenty-three diploid, six triploid ('Daisetsuzan', 'Goko', 'Horiuchikanzaki'*, 'Issho-noharu', 'Meicho', and 'Yuhime'*), nine tetraploid ('Ayaka'*, 'Eiko', 'Hoshuku'*, 'Hoshun', 'Sachi-no-haru'*, 'Shunka'*, 'Taihei', 'Taikonotsuki'*, and R. kiusianum x R. eriocarpum No. 5) and four mixaploid ('Koyo', 'Miharu'*, 'Shinsen', and 'Sulsen'*) evergreen azaleas and eight diploid and five tetraploid ('Golden Flare', 'Golden Sunset', 'Klondyke', 'Melford Yellow', and R. japonicum f. flavum No. 6) deciduous azaleas. Although flow cytometry can be used to directly compare relative genome sizes of tissue from related taxa, inclusion of an internal standard with a known genome size allows the calculation of the sample genome size (Doležel and Bartoš, 2005), which enables comparisons among studies of more divergent taxa.
The objectives of this project were to determine the ploidy level and relative genome size of a diverse collection of species, hybrids, and cultivars of rhododendron by using a combination of flow cytometry and traditional cytology in order to: 1) determine the ploidy level of suspected, but unconfirmed, polyploid taxa (both naturally occurring and chemically induced), 2) increase sampling among and within species, and 3) develop an extensive database for specific cultivars and clones for use by rhododendron breeders.
Materials and Methods
Flow cytometry. Holoploid, 2C genome sizes (i.e., DNA content of the entire non-replicated, chromosome complement irrespective of ploidy level) were determined via flow cytometry (de Laat et al., 1987; Doležel, 1991; Galbraith et al., 1983; Greilhuber et al., 2005). Diverse species and cultivars were acquired from various sources that included taxa from the Hymenanthes , Rhododendron , Tsutsusi , and Pentanthera subgenera along with several inter-subgeneric hybrids (Table 1). Approximately 1 cm 2 of newly expanded leaf or petal tissue was finely chopped with a razor blade in a Petri dish with 500 mL of nuclei extraction buffer (CyStain UV Precise P Nuclei Extraction Buffer, Partec, Münster, Germany). The solution was incubated for 1 to 2 min at approximately 24 °C and then filtered through Partec CellTrics™ disposable filters with a pore size of 50 mm to remove tissue debris. Nuclei were stained with 1.5 mL 4', 6-Diamidino-2-phenylindole (DAPI) staining buffer (CyStain UV Precise P Staining Buffer, Partec). Stained nuclei were analyzed with a flow cytometer (Partec PA-I, Partec) to determine relative genome size. Counts exceeded a minimum of 3000 cells per sample. Genome sizes were determined by comparing mean relative fluorescence of each sample with an internal standard, Pisum sativum L. 'Ctirad', with a known genome size of 9.09 pg (Bennett and Smith, 1976; Doležel et al., 1998) and calculated as: 2C genome size of sample = 9.09 pg x (mean fluorescence value of sample/mean fluorescence value of standard). The relationship between ploidy levels and genome sizes was initially determined for plants with documented chromosome numbers including diploid R. 'Fragrant Affinity', triploid R. 'Redwing' azalea, and the tetraploid Ilam azalea #HA L49-520 (Contreras et al., 2007; De Schepper et al., 2001; Krebs, 1997). Genome sizes were also determined for a range of species where ploidy levels and chromosome counts have been previously reported. Mean 1Cx monoploid genome size (i.e., DNA content of the non-replicated base set of chromosomes with x = 13) was calculated as 2C genome size / ploidy level. Data were subjected to analysis of variance and means separation by using the Waller procedure (PROC GLM; SAS version 8.02, SAS Institute., Cary, N.C.; SAS Institute, 1988).
Chromosome counts. In situations where cytometric results were not consistent with published research, chromosomes were counted by using standard cytological techniques (Contreras et al., 2007). Chromosomes were counted in mitotic cells from young root tips of rhododendron cuttings. Roots were collected before 11 a.m. and root tips were placed in a pre-fixative solution of 2mM 8-hydroxyquinoline for 4 hours at 12 °C in the dark. Root tissue was fixed in a 1 : 3 solution of propionic acid : 95% ethanol solution for 24 hours at room temperature and then hydrolyzed in 1N HCl for 15 minutes at room temperature and for 25 minutes at 60 °C, followed by a rinse in distilled water. Root tips were excised and placed on a glass microscope slide with a drop of 1% acetocarmine. Slides with tissue samples were heated to approximately 70°C for 10 to 15 s, squashed with a coverslip, and viewed under a light microscope (Nikon Eclipse 80i, Nikon, Melville, NY) at 1,500x using oil immersion.
Results and Discussion
Flow cytometry was an effective method for determining genome sizes and ploidy levels of rhododendron. Mean 2C holoploid genome sizes varied as a function of subgenus and ploidy level (Tables 1 and 2). Analysis of variance demonstrated significant effects of both subgenus and ploidy level on 2C genome size (P<0.05). Genome sizes (2C) within ploidy levels for a given subgenus had a narrow range providing clear distinction among ploidy levels. Mean 1Cx monoploid genome size was conserved across ploidy levels within a subgenus, ranging from 0.72 to 0.75 pg for subgenus Hymenanthes , 0.67 to 0.83 pg for subgenus Rhododendron , 0.63 to 0.67 pg for subgenus Tsutsusi , and 0.80 to 0.83 for subgenus Pentanthera (Table 2). There did not appear to be a consistent reduction in base 1Cx genome size with increasing ploidy level (i.e, genome downsizing) in rhododendron as has been commonly found in other genera with polyploid series (Leitch and Bennett, 2004). These results were based on cytometry methods using DAPI staining that provides consistent determination of relative genome size. However, it should be noted that other methods and stains may provide slightly different values and ranges (Doležel and Bartoš, 2005).
| Table 2 . Summary of means and ranges for 2C, holoploid genome size (ρg) and 1Cx monoploid genome size (pg) by subgenus and ploidy level. | |||||
| Subgenus | Ploidy level | ||||
| Diploid (2x) | Triploid (3x) | Tetraploid (4x) | Hexaploid (6x) | Octoploid (8x) | |
| Hymenanthes |
2C = 1.50 ± 0.01 A
(1.41-1.64) 1Cx = 0.75 ± 0.01 A (0.71-0.82) |
2C = 2.17 ± 0.05 B
(2.06-2.22) 1Cx = 0.72 ± 0.02 A (0.69-0.74) |
2C = 3.01 ± 0.04 C
(2.89-3.37) 1Cx = 0.75 ± 0.01 A (0.72-0.84) |
NA | NA |
| Rhododendron |
2C = 1.65 ± 0.05 A
(1.32-1.86) 1Cx = 0.83 ± 0.02 A (0.66-0.93) |
2C = 2.01 ± -- B
(NA) 1Cx = 0.67 ± -- B (NA) |
2C = 3.06 ± 0.05 C
(2.78-3.25) 1Cx =0.77 ± 0.01 AB (0.70-0.81) |
2C = 4.48 ± 0.04 D
(4.39-4.61) 1Cx = 0.75 ± 0.01 AB (0.73-0.77) |
5.70 ± 0.28 E
(5.42-5.97) 1 Cx = 0.72 ± 0.03 AB (0.68-0.75) |
| Pentanthera |
2C = 1.62 ± 0.01 A
(1.51-1.74) 1Cx = 0.81 ± 0.01 A (0.76-0.87) |
2C = 2.48 ± 0.06 B
(2.30-2.60) 1Cx = 0.83 ± 0.02 A (0.77-0.87) |
2C = 3.23 ± 0.02 C
(3.00-3.88) 1Cx = 0.81 ± 0.00 A (0.75-0.97) |
NA |
2C = 6.40 ± .03 D
(6.32-6.46) 1Cx =0.80 ± 0.00 A (0.79-0.81) |
| Tsutsusi |
2C = 1.26 ± 0.01 A
(1.22-1.30) 1Cx = 0.63 ± 0.01 A (0.61-0.65) |
2C = 1.93 ± 0.03 B
(1.88-1.98) 1Cx = 0.65 ± 0.01 AB (0.63-0.66) |
2C = 2.68 ± 0.08 C
(2.60-2.75) 1Cx = 0.67 ± 0.02 B (0.65-0.68) |
NA | NA |
| 1 Values represent means ± SEM followed by (ranges) derived from Table 1. Means followed by different letter, within a row, are significantly different, P < 0.05. | |||||
Hymenanthes
. Genomic sizes (2C) in this subgenus ranged from 1.4 to 1.6 pg for
diploids, from 2.1 to 2.2 pg for triploids, and from 2.9 to 3.4 pg for tetraploids
(Table 2). As expected from earlier reports (Ammal et al., 1950; Nakamura, 1931), all of
the sampled species fell within the diploid group (Table 1). However, some hybrids
derived from species within this subgenus exhibited polyploidy. Barlup (2002) speculated
on the possible polyploid nature of 'Taurus', ('The Honourable Jean Marie de Montague'
x
R. strigillosum
) and we found it to be triploid, which most likely explains its
low fertility. 'Hallelujah' ('The Honourable Jean Marie de Montague' x 'Kimberly') and an
unnamed hybrid [('Nancy Evans' × ('Whopper' × 'Lem's Cameo')) x 'Point Defiance'] were
also found to be triploids. These triploids may have arisen from either interploid crosses
(particularly when the tetraploid 'Point Defiance' was a parent) or from an unreduced
gamete from a diploid parent. Hybridity has been shown to increase formation of unreduced
gametes even when the parental species might not exhibit the same characteristic (Ramsey
and Schemske, 1998; Widrlechner et al. 1982). Other tetraploids arising from interspecific
hybridization in this subgenus included 'Horizon Monarch' ('Nancy Evans x 'Point Defiance'),
'Lem's Monarch' ('Anna' x 'Marinus Koster'), 'Point Defiance' ('Anna' x 'Marinus Koster'),
and 'Gentle Giant' ('Point Defiance' x 'Platinum Pearl'). 'Vulcan' tetraploid arose as
somatic mutation (i.e., branch sport) on 'Vulcan' (Harold Greer, Eugene, Ore., per. comm.).
Interestingly, we found 'Vulcan' tetraploid to be a 2x + 4x mixaploid that apparently arose
from a mitiotic doubling event within a single histogenic layer.
Several chemically-induced tetraploids were also confirmed including 'Everlasting Tetra'*,
'Supernova', 'Briggs Red Star', and
R. fortunei
(NCSU 2005-175). 'Everlasting Tetra'*
was developed from 'Everlasting' ('No Suchianum') (see Grant et al., 2004 for more history
on this cultivar) at N.C. State University based on methods described by Contreras et al.
(2007). 'Supernova' resulted from in-vitro colchicine treatment of 'Nova Zembla' at Briggs
Nursery, Olympia, Wash. (Dan Meier, Olympia Wash., per. comm.). 'Briggs Red Star' was
developed similarly at Briggs Nursery, but was found to be a 2x + 4x mixapoloid.
R.
fortunei
NCSU 2005-175 was a colchicine treated plant developed by Dr. Max Byrkit,
Williamsport, Md. (Kehr, 1996 b).
Rhododendron
. Concordant with previous findings, polyploidy was prevalent among
species and their hybrid derivatives from subgenus
Rhododendron
(Ammal et al., 1950).
Genome sizes (2C) for diploids ranged from 1.3 to 1.9 pg, there was one triploid at 2.0 pg,
tetraploids ranged from 2.8 to 3.3 pg, and hexaploids ranged from 4.4 to 4.6 pg (Table 2).
The relationship between genome size and ploidy level above the hexaploid level was less
clear. Two
R. maddenii
clones had genome sizes ranging from 5.4 to 5.8 pg that are
most likely octoploids, but the plant with 5.4 pg could possibly be heptaploid. The only
triploid found was 'White Ruffles', a cross made by Dr. August Kehr between the tetraploid
R. carolinianum
'Epoch' (Kehr, 1996b) and
R. mucronulatum
.
Rhododendron
augustinii
was found to be tetraploid as reported previously (Ammal et al., 1950)
as were Dr. Kehr's
augustinii
hybrids: 37-1, 37-4, and 37-7 (Dr. Kehr, per. comm.).
'Shorty', a cross between a selfed 'Epoch' and 'Hi Tech' (Henry Schannen, Jackson,
N.J., per. comm.), was a tetraploid indicating that 'Hi Tech' is either a tetraploid or
produced unreduced pollen. 'Bubblegum' and 'Northern Starburst' were both tetraploids
and were developed from in-vitro colchicine treatment of 'Weston's Aglo' and PJM Group
respectively, at Briggs Nursery (Dan Meier, Olympia Wash., per. comm.).
Pentanthera
. Genome sizes for species and hybrids in subgenus
Pentanthera
ranged
from 1.5 to 1.7 pg for diploids, 2.3-2.6 for triploids, 3.0-3.9 for tetraploids, and 6.3-6.5
for octoploids. The majority of deciduous azaleas, including
R. arborescens
,
alabamense
,
canescens
,
cumberlandense
,
periclymenoides
,
prinophyllum
,
prunifolium
,
serrulatum
,
vaseyi
, and
viscosum
were found to be diploids as has been reported previously (Ammal, 1950; Li, 1957; Sax, 1930).
The more recently discovered
R. eastmanii
was also found to be a diploid (Kron and Creel,
1999). Also agreeing with past literature (Ammal et al., 1950; Li, 1957; Sax, 1930) was the
confirmation of
R. calendulaceum
as a tetraploid, though one triploid
R.
calendulaceum
, NCSU 2000-164, was found that most likely resulted from a natural hybrid
with a diploid species. Three wild-collected accessions of Gregory Bald Hybrids were found
to be diploids, confirming that their parentage does not include the tetraploid
R. calendulaceum
.
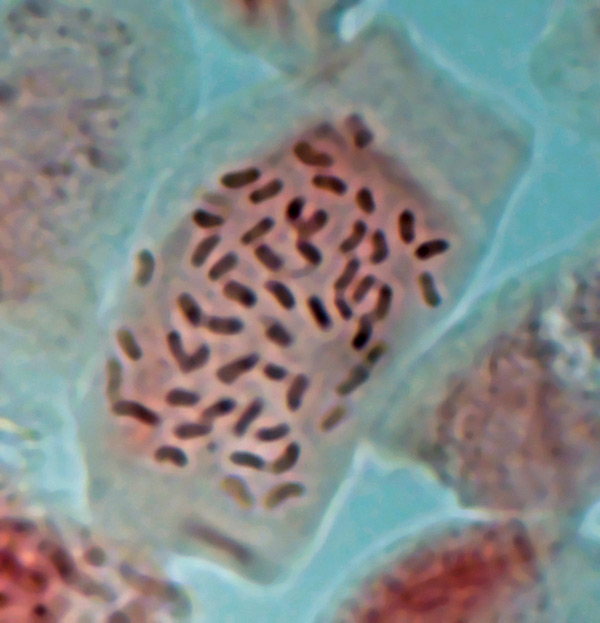
|
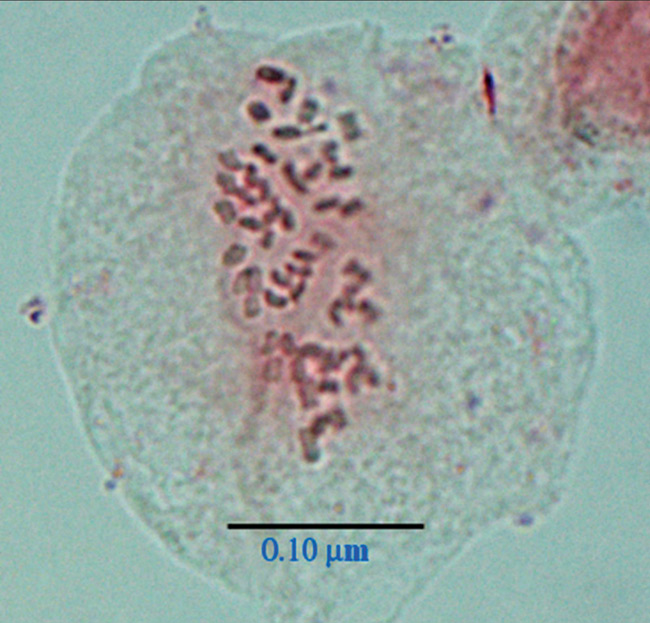
|
|
|
Figure 1. Photomicrograph of root tip cell of
R. austrinum
(2006-223)
in prophase with 2n = 4x = 52 somatic chromosomes. |
Figure 2. Photomicrograph of root tip cell of
R. atlanticum
(H2004-054-002)
in prophase with 2n = 4x = 52 somatic chromosomes. |
Our cytometric evidence suggests that natural polyploidy may be more prevalent among
deciduous azalea species than previously thought. The data obtained for two selections of
the Pontic azalea,
R. luteum
'Bumb'* and 'Golden Comet' (Table 1) substantiate a
finding by Eeckhaut et al. (2004) that this Central Asian species has tetraploid forms.
All of the
R. atlanticum
and
R. austrinum
accessions tested in this study
(Table 1) had polyploid genome sizes (mostly tetraploid and a few triploid), as did some
of the
R. flammeum
and
R. occidentale
samples. This is notable because in
all earlier reports, only one instance of polyploidy (triploid) in these four North
American species has been reported (Ammal, 1950; Li, 1957; Sax, 1930). Cytometric results
in the present study were confirmed by chromosome counts on somatic cells from fifteen
accessions of both
R. atlanticum
and
R. austrinum
, which showed that they were
tetraploids, 2n = 4x = 52 (Figs. 1 and 2). Indirect evidence of tetraploidy in
R. atlanticum
is provided by the observation that
R. atlanticum
H2004-055
and H2004-056 readily hybridize with
R. calendulaceum
and produce fertile hybrids
(Dr. Jim Ballington, N.C. State University, Raleigh, N.C., per. comm.). Fertile hybrids
have also resulted from crosses between
R. calendulaceum
x
austrinum
,
R. calendulaceum
x
atlanticum
,
R. calendulaceum
x 'Marydel' (Mr.
Ray Head, Rutherfordton, N.C., per. comm.).
No diploid
R. austrinum
or
R. atlanticum
was found despite extensive
sampling of taxa from diverse sources and geographical origins (26
R. austrinum
and 30
R. atlanticum
accessions collected throughout the Southeast). The
assessment of these species as diploids in previous studies was based on a much
more limited sampling (Ammal, 1950; Li, 1957; Sax, 1930). Therefore it seems unlikely
that the lack of diploid forms of
R. atlanticum
and
R. austrinum
in this
survey represents a sampling limitation, but rather a predominance of polyploids in
these species. This appears to be the case for
R. calendulaceum
as well, where
there are no reports (present study included) of diploid populations. The two triploid
R. austrinum
accessions observed here (Table 1) may have resulted from a natural
interploid cross between sympatric diploid and tetraploid populations - if so it would
be informative to sample again from the areas where they were collected in order to
document the presence of more diploid forms of this species.
The best example of a natural polyploid series in
Rhododendron
species appears
to be
R. occidentale
, where both diploid and tetraploid accessions were observed
(Table 1). These data suggest there is a range of ploidy levels found within this
species as is naturally found in many other species, e.g., Galax aphylla (Nesom, 1983),
representing an evolutionary progression (Arnold, 1997; Briggs and Walters, 1997).
Multiple ploidy levels were also observed for
R. flammeum
and
R. flammeum
hybrids. However, since
R. calendulaceum
can appear very similar to
R.
flammeum
, additional sampling from wild populations would be desirable to confirm
this finding.
Many hybrid cultivars within this subgenus were found to be polyploids; most likely
resulting from the hybridization of polyploid parents. Three Exbury azaleas of unknown
parentage, 'Gibraltar', 'Gold Dust', and 'Klondyke', were tetraploids as were 'My Mary'
('Nacoochee' x 'Austrinum Gold'), 'Lemon Lights' (Northern Lights Series, unknown
parentage), 'Admiral Semmes'* (Confederate Series, 'Hotspur Yellow' x
R. austrinum
),
'Marydel' (
R. atlanticum
or possible hybrid with
R. periclymenoides
) and
an unnamed Ilam hybrid (HA L-46-520; unknown parentage) (Dirr, 1998; Galle, 1987).
'Snowbird' was determined to be a tetraploid and is believed to be a natural hybrid
between
R. atlanticum
and
R. canescens
(Galle, 1987), suggesting an
unreduced gamete from the
R. canescens
parent. 'Fragrant Star', developed through
in-vitro colchicine treatment of 'Snowbird' at Briggs Nursery (Dan Meier, Olympia Wash.,
per. comm.) was found to be an octoploid as were open pollinated (selfed) seedlings
from 'Fragrant Star'.
Tsutsusi . The ranges for 2C genome sizes in subgenus Tsutsusi were consistently lower than the other subgenera with the diploids ranging from 1.2 to 1.3 pg, the triploids from 1.9 to 2.0 pg, and the tetraploids from 2.6 to 2.8 pg. We found 'Red Wing' to be a triploid which was consistent with the findings of Pryor and Frazier (1970), but contrary to the findings of Heursel and Roo (1981), who found it to be a diploid, suggesting that multiple clones may exist under the same name. The purple-leaved 'Crimson Majesty'*, a sport of 'Red Formosum' was also found to be a triploid as was an unnamed hybrid between 'Pink Gloria Tetra' x 314-1 (NCSU 2000-171). The clone 314-1, a colchicine-treated seedling (open-pollinated seedling of 'Perle de Swynaerde' x 'Pryor Dwarf'*) developed by Dr. August Kehr, was also found to be a tetraploid, as was the unnamed hybrid 'Anytime Tetra' x 314-1 (NCSU 2000-167). We did not have access to 'Pink Gloria Tetra' and could not determine its ploidy. However, upon further investigation, we found the original 314-1 specimen, provided by Dr. Kehr, to be a mixture of diploid and tetraploid shoots with diploid shoots arising from below the treated crown. If flowers from these diploid shoots were used in breeding with the presumed tetraploid 'Pink Gloria Tetra', a triploid could have resulted.
Inter-subgeneric Hybrids . Several hybrids were examined that were the result of crosses between subgenera. In agreement with Contreras et al. (2007), we confirmed that 'Fragrant Affinity'* ( R. viscosum x R. ponticum ) was a diploid and its allopolyploid complement, 'Fragrant Affinity Tetra',* was a tetraploid. The hybrids R. calendulaceum x '314-1' and 'Briggs Red Star' x 'Fragrant Affinity Tetra' were tetraploids as expected given that all parents were also tetraploids.
| Table 1 . Relative genome size and estimated ploidy level, determined by flow cytometry, for a diverse collection of rhododendron species and cultivars. | |||
| Taxa | Source 1 |
Relative 2C
genome size (pg) 2 |
Estimated
ploidy (x) |
|
Subgenus Hymenanthes
Species |
|||
|
catawbiense
'Catalgla'
fortunei maximum maximum ponticum (variegated) sinogrande |
HA
NCSU 2003-144 NCSU 2006-281 NCSU 2005-243 NCSU 2006-047 NCSU 2006-038 |
1.44±0.03
1.55±0.02 1.44±0.01 1.53±0.12 1.46±0.01 1.54±0.04 |
2
2 2 2 2 2 |
| Hybrids | |||
|
'Cheyenne'
'Everlasting'* 'Fantastica' 'Goldflimmer' 'Janet Blair' 'Maxecat' 'Nova Zembla' 'Polar Bear' 'Puget Sound' 'Queen Anne's' x 'Gold Dust' 'Vulcan' 'Vulcan's Flame' 'Taurus' 'Hallelujah' [Nancy Evans x (Whopper x Lem's Cameo)] x Point Defiance 'Gentle Giant' 'Grand Slam' 'Horizon Monarch' 'Horizon Monarch' x 'Point Defiance' (clone R) 'Lem's Monarch' 'Point Defiance' 'Vulcan Tetraploid'* |
NCSU 2002-086
NCSU 2000-162 NCSU 2004-285 JCRA 040681 NCSU 2004-291 NCSU 2005-238 NCSU 2006-093 NCSU 2002-089 NCSU 2005-015 NCSU 2000-270 NCSU 2006-095 NCSU 2004-134 NCSU 2006-026 NCSU 2005-009 Brockenbrough NCSU 2006-020 NCSU 2006-021 NCSU 2006-022 Brockenbrough Brockenbrough Brockenbrough NCSU 2004-103 |
1.41±0.03
1.52±0.03 1.45±0.00 1.64±0.01 1.44±--- 1.52±0.00 1.53±0.01 1.55±0.02 1.47±0.00 1.43±0.02 1.49±0.03 1.55±0.01 2.06±0.06 2.22±0.05 2.22±0.06 3.37±0.11 3.03±0.02 2.89±.0.07 2.93±0.00 2.90±0.01 2.93±0.02 1.51±0.02 3.03±0.07 |
2
2 2 2 2 2 2 2 2 2 2 2 3 3 3 4 4 4 4 4 4 2+4 |
| Induced polyploids | |||
|
'Briggs Red Star'
'Everlasting Tetra'* 'Supernova' fortunei |
NCSU 2002-260
NCSU 2005-149 NCSU 2002-263 NCSU 2005-175 |
1.53±0.02
3.04±0.05 2.86±0.02 2.98±0.04 3.14±0.03 |
2+4
4 4 4 |
|
Subgenus Rhododendron
Species |
|||
|
edgeworthii
'Bodnant'*
edgeworthii 'Ice'* augustinii maddenii maddenii maddenii subsp. crassum maddenii subsp. maddenii maddenii maddenii subsp. crassum |
NCSU 2005-361
NCSU 2006-053 NCSU 2000-170 NCSU 2006-162 NCSU 2006-160 NCSU 2006-256 NCSU 2006-037 NCSU 2006-161 NCSU 2006-258 |
1.75±0.01
1.76±0.04 3.10±0.01 4.41±0.04 4.45±0.02 4.39±0.01 4.47±0.01 5.97±0.01 5.42±0.01 |
2
2 4 6 6 6 6 8 8 |
| Hybrids | |||
|
'Aglo'
'April Rose' 'California Gold' 'Coastal Spice' 'Dora Amateis' 'Improved Fragrantissimum'* 'McNabii' 'Mysterious Maddenii'* PJM Group 'Reine Long' 'Southern Cloud' 'White Ruffles'* 'Blue Target' 'Epoch' x augustinii 'Gletschernacht' 37-1 37-4 37-7 'Shorty' 'Bernice' 'Pink Trumpets'* |
NCSU 2006-045
NCSU 2006-018 NCSU 2006-259 NCSU 2005-355 NCSU 2005-222 NCSU 2002-088 NCSU 2006-039 NCSU 2006-262 NCSU 2006-012 NCSU 2006-264 NCSU 2006-265 NCSU 2006-113 NCSU 2000-168 NCSU 2006-044 NCSU 2003-143 NCSU 2000-267 NCSU 2000-169 NCSU 2000-269 NCSU 2006-042 NCSU 2006-255 NCSU 2006-263 |
1.49±0.05
1.36±0.02 1.71±0.02 1.86±0.01 1.62±0.06 1.72±0.05 1.61±0.02 1.82±0.00 1.32±--- 1.76±0.02 1.65±0.04 2.01±0.03 3.10±0.00 3.25±0.02 2.78±0.07 3.11±0.02 3.12±0.10 3.19±0.00 3.22±0.04 4.57±0.01 4.61±0.02 |
2
2 2 2 2 2 2 2 2 2 2 3 4 4 4 4 4 4 4 6 6 |
| Induced polyploids | |||
|
'Bubblegum'
'Northern Starburst' |
NCSU 2006-046
NCSU 2006-011 |
2.90±0.07
2.81±--- |
4
4 |
|
Subgenus Pentanthera
Species |
|||
|
alabamense
arborescens austrinum (OP) austrinum (pale yellow) canescens 'Crains Creek'* canescens 'Sp. Found'* canescens 'White Canescens'* cumberlandense eastmanii (Newberry, SC) eastmanii (York Co, SC) flammeum (ES Selection) flammeum occidentale 'Humboldt Picotee' occidentale 'Tatum's Deep Pink'* periclymenoides (nudiflorum) prinophyllum prunifolium prunifolium serrulatum vaseyi viscosum austrinum austrinum 'Firecracker'* calendulaceum atlanticum atlanticum atlanticum atlanticum atlanticum atlanticum atlanticum atlanticum atlanticum atlanticum atlanticum atlanticum atlanticum atlanticum atlanticum atlanticum #1 atlanticum #1 (Del Mar Pen.) atlanticum #2 atlanticum #2 atlanticum #3 atlanticum #3 atlanticum #4 atlanticum #4 atlanticum #5 atlanticum #6 atlanticum #7 atlanticum #8 atlanticum 'Choptank Pink &White'* atlanticum atlanticum 'Winterthur'* austrinum austrinum austrinum austrinum austrinum austrinum austrinum austrinum austrinum austrinum austrinum austrinum austrinum austrinum #1 (Nat. For. Ala.) austrinum #10 austrinum #12 austrinum #2 austrinum #3 austrinum #4 austrinum #5 austrinum #6 austrinum 'Austrinum Gold'* austrinum 'Flame'* austrinum 'Millie Mac' calendulaceum 'Deliverance'* calendulaceum flammeum flammeum 'Pink Surprise'* luteum 'Bumb'* luteum 'Golden Comet' occidentale 'Double Dig Twelve' |
2004-114
NCSU 1998-454 BE BE TNCA 1995-26*B TNCA 1989-60*A TNCA 1989-59*A TNCA 1994-10*B Cantrell Cantrell TNCA 1994-454*B TNCA 1995-31*B Cavender Cavender NCSU 2000-419 TNCA 1994-20*A TNCA 1994-22*B NCSU 1998-455 TNCA 1989-78*A NCSU 1998-447 TNCA 1995-460*A TNCA 1989-40*A TNCA 1995-451*A NCSU 2000-164 JCRA 050431 TNCA 1998-0103a NCBG 1994-0093b NCBG 1986-2041a NCSU H2004-054-002 TNCA 1994-9*B NCSU H2004-056-002 NCSU H2004-055-003 NCSU H2004-055-001 NCSU H2004-056-001 NCSU H2004-055-002 NCSU H2004-054-004 NCSU H2004-054-001 NCSU H2004-054-003 TNCA 1989-33*A HA HA HA HA HA HA HA HA HA HA HA HA TNCA 1995-467*B TNCA 1998-34*A JCRA 000609 JCRA 020494 NCBG 1991-0301a TNCA 1996-0374a JCRA L20 TNCA 1994-339*B TNCA 1989-221*E NCBG 1998-0104a TNCA 1989-221*A NCBG 1998-0188a NCSU 2005-062 NCSU 2006-223 NCSU 2004-117 NCSU 2005-063 HA HA HA HA HA HA HA HA TNCA 1989-38*A TNCA 1990-22*A TNCA 1993-327*A TNCA 1989-55*A NCSU H2000-048 NCSU 2007-001 TNCA 1994-332*B NCSU 2005-101 NCSU 2006-006 Cavender |
1.66±0.05
1.65±0.05 1.59±0.00 1.64±0.02 1.72±0.02 1.61±0.01 1.65±0.01 1.63±0.02 1.58±0.04 1.60±0.04 1.68±0.01 1.72±0.02 1.51±0.04 1.51±0.06 1.68±0.00 1.64±0.00 1.56±0.01 1.58±0.00 1.74±0.03 1.56±0.01 1.67±0.04 2.52±0.05 2.48±0.01 2.30±0.07 3.01±0.01 3.05±--- 3.10±--- 3.12±--- 3.15±0.01 3.16±0.00 3.20±0.00 3.20±0.02 3.21±0.01 3.24±0.00 3.24±0.02 3.24±0.07 3.26±0.00 3.26±0.01 3.27±0.00 3.16±0.02 3.13±0.06 3.10±--- 3.33±0.02 3.10±0.01 3.29±0.01 3.12±0.03 3.14±0.01 3.08±.00 3.06±--- 3.19±0.05 3.32±--- 3.22±0.01 3.20±0.02 3.18±0.02 3.11±0.03 3.12±--- 3.21±--- 3.21±0.05 3.24±0.01 3.43±0.03 3.47±--- 3.41±0.01 3.31±--- 3.33±0.03 3.34±0.04 3.36±0.01 3.37±0.02 3.33±0.00 3.37±0.09 3.30±0.00 3.23±0.01 3.27±0.05 3.88±0.59 3.32±0.00 3.29±0.02 3.28±0.01 3.28±0.01 3.33±0.07 3.28±0.01 3.14±0.09 3.14±0.03 3.24±0.03 3.00±0.01 3.00±0.01 2.94±.08 |
2
2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 3 3 3 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 |
| Hybrids | |||
|
'August Beauty'*
'Lemon Drop' 'Millennium' 'Popcorn' 'Summer Lyric' 'Weston's Parade'* Gregory Bald Hybrid Gregory Bald Hybrid Gregory Bald Hybrid flammeum x canescens 'Admiral Semmes'* flammeum x calendulaceum 'Gilbralter' 'Gold Dust' 'Klondyke' 'Lemon Lights' 'Marydel' 'My Mary' 'Snowbird' Ilam hybrid |
NCSU 2006-118
NCSU 2006-119 NCSU 2005-122 NCSU 2005-123 NCSU 1998-453 NCSU 2005-121 TNCA 1992-515*M TNCA 1992-212*E TNCA 1992-213*B TNCA 1994-2*F NCSU 2005-081 TNCA 1996-325*A NCSU 2005-356 NCSU 2005-111 NCSU 2005-357 NCSU 2005-113 NCSU 1998-456 NCSU 2006-117 NCSU 2006-048 HA L49-520 |
1.58±0.00
1.51±0.04 1.61±0.03 1.51±0.01 1.64±0.04 1.57±0.06 1.62±0.03 1.65±0.01 1.67±0.03 2.60±0.05 3.15±0.04 3.39±0.01 3.38±0.01 3.27±0.04 3.26±0.16 3.03±0.00 3.43±0.03 3.15±0.08 3.24±0.03 3.17±0.01 |
2
2 2 2 2 2 2 2 2 3 4 4 4 4 4 4 4 4 4 4 |
| Induced polyploids | |||
|
'Fragrant Star'
'Fragrant Star' selfed 'Fragrant Star' selfed 'Fragrant Star' selfed |
NCSU 2004-293
NCSU H2006-007-003 NCSU H2006-007-001 NCSU H2006-007-004 |
6.32±0.03
6.39±0.11 6.41±0.08 6.46±0.03 |
8
8 8 8 |
|
Subgenus Tsutsusi
Species |
|||
| stenopetalum 'Linearifolium' | JCRA 050534 | 1.27±0.01 | 2 |
| Hybrids | |||
|
'Conles' Autumn Express ™
'Glacier' 'Hardy Gardenia'* 'Polar Bear' 'Secret Wish' 'Crimson Majesty'* 'Pink Gloria Tetra' x '314-1' 'Redwings' 'Anytime Tetra'* × '314-1' |
NCSU 2002-237
NCSU 2005-064 NCSU 2005-023 NCSU 2005-196 NCSU 2005-097 NCSU 2004-245 NCSU 2000-171 NCSU 2006-094 NCSU 2000-167 |
1.27±0.02
1.24±0.03 1.22±0.00 1.26±0.00 1.30±0.01 1.94±0.03 1.98±0.01 1.88±0.02 2.75±0.07 |
2
2 2 2 2 3 3 3 4 |
| Induced polyploids | |||
| '314-1' | NCSU 2000-165 | 2.60±0.01 | 4 |
|
Inter-Subgeneric
Hybrids |
|||
|
'Fragrant Affinity'*
'Briggs Red Star' x 'Fragrant Affinity Tetra'* R. calendulaceum x '314-1' |
NCSU H2003-003
NCSU H2005-085 NCSU H2006-008-001 |
1.59±0.12
2.99±0.03 2.81±.00 |
2
4 4 |
| Induced polyploids | |||
| 'Fragrant Affinity Tetra'* | NCSU H2003-002 | 3.11±0.04 | 4 |
|
* Name is not registered.
1 BE – Biltmore Estate, Asheville, N.C. Brockenbrough – Mr. Ned Brockenbrough, Hunts Point, Wash. Cantrell – Mr. Allen Cantrell, Chesnee, SC. Cavender – Mr. Dick 'Red' Cavender, Sherwood, Oregon. HA = Holden Arboretum, Kirtland and Madison, Ohio. TNCA = The North Carolina Arboretum, Asheville, N.C. NCBG = North Carolina Botanical Garden, Chapel Hill, NC. NCSU = North Carolina State University, Mountain Horticultural Crops Research and Extension Center, Fletcher, N.C. 2 Values represent mean 2C holoploid genome size ± SEM for two samples. Values with no SEM indicate only one sample was analyzed. |
|||
Conclusion
This study provides extensive information on genome sizes and ploidy levels for abroad
range of species, cultivars, and hybrids of rhododendron including naturally occurring
and induced polyploids. Flow cytometry was an efficient and effective method for
determining genome size of rhododendron. Genome sizes (2C) within ploidy levels for a
given subgenus had a narrow range providing clear distinction among ploidy levels.
Polyploidy was found to be common in the genus
Rhododendron
and considerably
more prevalent in the subgenus
Pentanthera
than previously known. Particularly
noteworthy were the findings that
R. occidentale
includes both diploid and
tetraploid individuals and that
R. atlanticum
and
R. austrinum
are
predominantly tetraploid species. This information provides further insights into
the genetics, evolution, and reproductive biology of rhododendron as well as serving
as a valuable database for breeders.
Literature Cited
Ammal, E.K.J. 1950. Polyploidy in the genus rhododendron.
Rhododendron Year Book
.
5:92-98.
Ammal, E.K.J., I.C. Enoch, and M. Bridgwater. 1950. Chromosome numbers in species of
rhododendron.
Rhododendron Year Book
. 5:78-91.
Arnold, M.L. 1997.
Natural Hybridization and Evolution
. Oxford Univ. Press., N.Y.
Atkinson, R., K. Jong, and G. Argent. 2000. Chromosome numbers of some tropical rhododendrons
(section
Vireya
).
Edinb. J. Bot
. 57:1-7.
Barlup, J. 2002. Let's talk hybridizing: Hybridizing with elepidote polyploid rhododendrons.
J. Am. Rhod. Soc
. 76:75-77.
Bennett, M.D. 2004. Perspectives on polyploidy in plants – ancient and neo.
Bio.
J. Linnean Soc
. 82:411-423.
Bennett, M.D. and J.B. Smith. 1976. Nuclear DNA amounts in angiosperms. Philosophical
Transactions of the Royal Society of London Series B-Biological Sciences 274:227-274.
Briggs, D. and S.M. Walters. 1997.
Plant Variation and Evolution
. 3rd ed.
Cambridge Univ. Press, Cambridge, U.K.
Chahal, G.S. and S.S. Gosal. 2002. Principles and procedures of plant breeding:
Biotechnological and conventional approaches. Alpha Sci. Inter., Pangbourne, U.K.
Chen, Z.J. and Z.F. Ni. 2006. Mechanisms of genomic rearrangements and gene expression
changes in plant polyploids.
BioEssays
28:240-252.
Contreras, R.N., T.G. Ranney, and S.P. Tallury. 2007. Reproductive behavior of diploid
and allotetraploid
Rhododendron
L. 'Fragrant Affinity'.
HortScience
42:31-34.
de Laat, A.M.M., W. Gohde, M.J.D.C Vogelzang. 1987. Determination of ploidy of single plants
and plant populations by flow cytometry.
Plant Breeding
99:303-307.
De Schepper, S., L. Leus, M. Mertens, E. Van Bockstaele, and M. De Loose. 2001. Flow cytometric
analysis of ploidy in
Rhododendron
(subgenus
Tsutsusi
).
HortScience
36:125-127.
Dirr, M.A. 1998.
Manual of Woody Landscape Plants: Their Identification, Ornamental
Characteristics, Culture, Propagation and Use
. 5th ed. Stipes Pub., Champaign, Ill.
Doležel, J. 1991. Flow cytometric analysis of nuclear DNA content in higher plants.
Phytochemical Analysis 2:143-154.
Doležel, J. and J. Bartoš. 2005. Plant DNA flow cytometry and estimation of nuclear genome
size.
Ann. Bot
. 95:99-110.
Doležel, J., J. Greihuber, S. Lucretti, A. Meister, M.A. Lysák, L. Nardi, R. Obermayer. 1998.
Plant genome size estimation by flow cytometry: Inter-laboratory comparison.
Ann. Bot
.
82 (Suppl. A):17-26.
Eeckhaut, T.G.R., L.W.H. Leus, A.C. De Raedt, and E.J. Van Bockstaele. 2004. Occurrence of
polyploidy in
Rhododendron luteum
Sweet, Hardy Ghent, and Rustica hybrids.
The Azalean
26:32-37.
Eiselein, J.E. 1994. An improved chromosome staining method applied to the study of colchicine
effects in
Rhododendron
.
J. Am. Rhod. Soc
. 48:143-146.
Galle, F.C. 1987.
Azaleas
: Revised and enlaged edition. Timber Press, Portland, Ore.
Galbraith, D.W., K.R. Harkins, J.M. Maddox, N.M. Ayres, D.P. Sharma, and E. Firoozabady.
1983. Rapid flow cytometric analysis of the cell cycle in intact plant tissues.
Science
220:1049-1051.
Grant, M.L., N.H. Toomey, and A. Culham. 2004. Is there such a thing as Kalmia x Rhododendron?
J. Amer. Soc. Hort. Sci
. 129:517-522.
Greilhuber, J., J. Doležel, M.A. Lysák, and M.D. Bennett. 2005. The origin, evolution and proposed
stabilization of the terms 'genome size' and 'C-value' to describe nuclear DNA contents.
Ann. Bot
. 95:255-260.
Heursel, J. and R. De Roo. 1981. Polyploidy in evergreen azaleas.
HortScience
16:
765-766.
Hosoda, T., A Moriya, and S. Sarahima. 1953. Chromosome numbers of satsuki,
Rhododendron
lateritium
P1.
Genetica
26:407-409.
Kehr, A.E. 1996a. Woody plant polyploidy.
Am. Nurseryman
183:38-47.
Kehr, A.E. 1996b. Polyploids in rhododendron breeding.
J. Am. Rhod. Soc
. 50:215-217.
Krebs, S.L. 1997. A cytogenetic study of sterile rhododendron hybrids.
J. Am. Rhod. Soc
.
51:70-74.
Kron, K.A. and M. Creel. 1999. A new species of deciduous azalea (Rhododendron section
Pentanthera; Ericaceae) from South Carolina.
Novon
9:377-380.
Leach, D.G. 1961.
Rhododendrons of the World and How to Grow Them
. Charles Scribners'
Sons, NY.
Leitch, I.J. and M.D. Bennett. 2004. Genome downsizing in polyploidy plants.
Bio. J. Linnean
Soc
. 82:651-663.
Li, H. 1957. Chromosome studies in the azaleas of eastern North America.
Amer. J. Bot
.
44:8-14.
Nakamura, M. 1931. Calyological studies on the genus Rhododendron.
J. Soc. Trop. Agric
.
Tohoku. Imp. Univ. 3:103-109.
Nesom, G., 1983.
Galax
(Diapensiaceae): Geographic variation in chromosome number.
Systematic Bot
. 8:1-14.
Paden, D.W., M.M. Meyer, Jr., and A.L. Raybum. 1990. Doubling of chromosomes with colchicine
treatment in vitro as determined by chloroplast number in epidermal guard cells.
J. Amer.
Rhod. Soc
. 44:162-165, 171.
Pryor, R.L. and L.C. Frazier. 1970. Triploid azaleas of the Belgian-Indian series.
HortScience
5:114-115.
Ramsey, J. and D.W. Schemske. 1998. Pathways, mechanisms, and rates of polyploidy formation
in flowering plants.
Annu. Rev. Ecol. Syst
. 29: 467-501.
Ranney, T.G. 2006. Polyploidy: From evolution to new plant development. Proc. Intern. Plant
Propagators' Soc. (in press).
Sakai, K., I. Miyajima, K. Ureshino, Y. Ozaki, and H. Okubo. 2004a. Oryzalin-induced
allotetraploids of an intersubgeneric hybrid between evergreen and deciduous azaleas.
J. Fac. Agri
. Kyushu Univ. 49:293-299.
Sakai, K, Y. Ozaki, M. Hiramatsu, A. Wakana, and H. Okubo. 2006. Intrasubgeneric and
interploid cross compatibility in evergreen and deciduous azaleas.
J. Fac. Agri
.
Kyushu Univ. 51:73-81.
Sakai, K., Y. Ozaki and H. Okubo. 2003. Intra and inter-ploid cross compatibility
among diploid triploid and tetraploid Satsuki azaleas.
J. Japan. Soc. Hort. Sci
. 72
(Suppl. 2): 205.
Sakai, K., Y. Ozaki, K. Ureshino, and I. Miyajima. 2004b. Effectiveness of inter-ploid
crosses for overcoming plastome-genome incompatibility in intersectional crosses of
azaleas.
Acta Hort
. 651(2):47-53.
SAS Institute Inc. 1988. SAS/STAT user's guide, release 6.03 edition. SAS Institute
Inc., Cary, NC.
Sax, K. 1930. Chromosome stability in the genus rhododendron.
Amer. J. Bot
.
17:247-251.
Soltis, D.E. and P.S. Soltis. 1993. Molecular data and the dynamic nature of polyploidy.
Critical Rev. Plant Sci
. 12:243-273.
Soltis, D.E., P.S. Soltis, and J.A. Tate. 2003. Advances in the study of polyploidy
since plant speciation.
New Phytol
. 161:173-191.
Soltis, P.S. and D.E. Soltis. 2000. The role of genetic and genomic attributes in
the success of polyploids.
Proc. Natl. Acad. Sci
. USA 97:7051-7057.
Thomas, H. 1993. Chromosome manipulation and polyploidy. In: M.D. Hayward,
N.O. Bosemark, and I. Rosmagosa (eds.)
Plant Breeding Principles and Prospects
.
Chapman and Hall, N.Y. 79-92.
Tolstead, W.L. and J.F. Glencoe. 1991. Winter-hardy tetraploids of
Rhododendron
carolinianum
and
Rhododendron racemosum
and their tetraploid hybrids.
J. Am. Rhod. Soc
. 45: 83-84.
Ureshino, K. and I. Miyajima. 1998. The relationship between appearance of albino seedlings
and their ploidy level in intersectional crossings among azalea species.
J. Japan.
Soc. Hort. Sci
. 67 (Suppl. 2):389.
Väinölä, A. 2000. Polyploidization and early screening of
Rhododendron
hybrids.
Euphytica
112:239-244.
Wendel, J.F. 2000. Genome evolution in polyloids.
Plant Mol. Bio
. 42:225-249.
Widrlechner, M., H. Pellett, P. Ascher. 1982. Unreduced gametes in azalea hybrids: a
possible breeding method for using promising azaleas of low fertility.
J. Am. Rhod.
Soc
. 36:98-100.
Acknowledgements
Appreciation is given to Tom Eaker, Joel Mowrey and the staff of the Mountain Horticultural Crops
Research Station for their excellent technical assistance. Thanks are also given to Mr. Ned
Brockenbrough, Hunts Point, Wash., Mr. Red Cavender, Sherwood, Oregon, Dr. Jim Ballington,
Raleigh, N.C., Mr. Allen Cantrell, Chesnee, S.C., Mr. Ray Head, Rutherfordton, N.C. and the staff
of the Holden Arboretum, Kirtland, Ohio, the North Carolina Botanical Garden, Chapel Hill,
N.C., The North Carolina Arboretum, Asheville, N.C., and the Biltmore Estate, Asheville, N.C.
for providing samples from their collections. Partial funding for this project was provided
by the Research Foundation of the American Rhododendron Society.
* Name is unregistered.
Jeff R. Jones is currently a masters student, Nathan P. Lynch is a research specialist, and Thomas G. Ranney is a professor of Horticultural Science and member of the Southeastern Chapter.
Stephen Krebs is a member of the Great Lakes Chapter.
