JARS v62n4 - But Why Is It Pink, Daddy? Part II
But Why Is It Pink, Daddy? Part II
Paul Rogers
Aloha, Oregon
Part I appeared in the Spring 2008 issue of the Journal.
I was getting to that. But before we can have pink, certainly we must have color. And since it's been a few months, let's review what we know about color in almost all flowers, and what we can surmise about color in rhododendrons.
Review of Part I
Enzymes synthesize most 1 of the complex molecules in living organisms, including flower pigments. The nature of enzymes is to be catalysts, to mediate biochemical reactions without being consumed by them. Enzymes are proteins, linear chains of amino acids. A gene specifies their order. That's all genes do, specify the sequences for building particular proteins. A handful of enzymes synthesize flavonoids from small molecules, which are raw materials for yellowish pigments. But they are also raw materials for another handful of competing enzymes that synthesize red, purple, and blue anthocyanin pigments. The final hue depends on whether any enzyme in either sequence fails or all of them work, and which win the competition. Flowering plants share the same genes that produce these enzymes. Where they differ is in accumulated mutations, or sports, that change the exact sequencing of the proteins. These variations in each gene result in alleles. They might be inconsequential (except in gene sequencing that shows lines of descent and taxonomy) or absolutely essential to the operation of the enzyme. For example, just one substitution in the right place causes human sickle-cell disease. In pigment synthesis, the effect is often to interrupt the synthesis pathway at the point the enzyme was supposed to act. In roses and rhododendrons for example, if F3'H doesn't work, chalcone precursors to yellow flavonoids accumulate rather than proceeding to red, purple, or bluish pigments of cyanidin. Conversely, if they are functional then synthesis proceeds through the other flavonoids to anthocyanins. Many species, usually in red to purple shades, do have white and yellow varieties explained this way. This is also good evidence the yellow flowers in elepidote rhododendrons are colored with flavonoids, not carotenes. Although carotenoids are yellow, so are flavonoids. Anthocyanin synthesis doesn't bother carotenes, and vice-versa. Each derives from different synthesis pathways of its own.
When hybridizing with yellow, knowing whether we're dealing with carotenoids or flavonoids is the first question we should answer. It's why making orange elepidotes isn't as easy as crossing red and yellow - a painter's eye for mixing colors will lead us astray. That could work if the yellow is carotenoid. For instance, one might assume the yellow of Rhododendron wardii is produced by a carotenoid; some literature says so. White and purple 'Peeping Tom' from R. wardii X 'Mrs. Furnivall' shows no yellow carotene. So the yellow must have been a flavonoid diverted to purple by functional enzymes from 'Mrs. Furnivall'.
By far, the most important secondary factor in the hue of red, purple, and blue anthocyanins is the pH of the petal tissue. The red rose and the blue cornflower have the same basic cyanidin pigment! (Cyan means blue, after all.) Tissue pH is controlled by a different set of genes than pigment. The general rule in anthocyanins is lower pH shifts hue toward red, higher pH shifts hue toward blue. Sometimes bicolors and blotches may add clues about effects of pH. Observations in the garden hint that pH might play a role in golden yellows too. Knowing how pigments are produced can illuminate our careful observations in the garden, and vice versa.
In a scant few sentences this may remind us of the previous article. Now, the previous article was "simplified," believe it or not, in order to present some version of the whole picture in reasonable space. This article is a follow-up and will presume everything in the other, especially Figure 1, without constant references. This time we'll explore making use of this information in hybridizing and point out some of the complications. Unfortunately, the science has been focused mostly on the anthocyanin pigment derivatives. We know less about the anthoxanthins, and much less about things like intensity. Still, some things are well established. Exploring what we know will inform us, and help illuminate our observations.
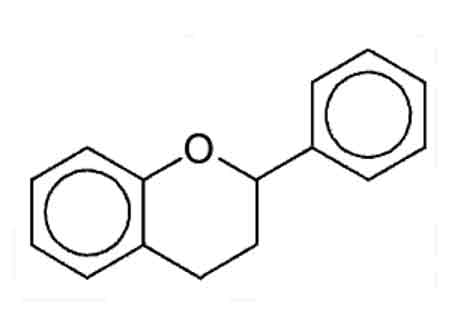
|
|
Figure 1. Flavylium substructure of flavonoids and anthocyanins.
Drawn by Paul Rogers |
Codominance and Incomplete Dominance
We should dismiss Mendel's concept of "dominant" and "recessive." Instead, we should understand it all in terms of whether the organism has functional enzymes or not. This correct interpretation carries us further.
The human ABO blood group gene shows this process clearly. We have a gene that mutated to produce antigens on the surface of red blood cells. Those who inherit this allele have enzymes that synthesize a specific antigen; hence "type A" blood. Those without have "type O." The gene mutated again to produce an allele specifying a different sequence in the enzyme, different antigens, and "type B" blood. If one inherits both alleles, one makes both antigens and has "type AB" blood. It's called "codominance," although there's no "dominance" about it. It's exactly what one would expect; the mutant alleles and their enzymes function independently. Neither actually "dominates" the allele that doesn't produce antigens, or each other. It's the immune system that says these are antigens, not the red blood cells. It just doesn't object to the cell surface proteins produced by "Type O."
The genes Mendel discovered in peas demonstrated simple dominant and recessive characteristics. White crossed with purple produced all purple progeny. They get the enzyme, synthesis proceeds, color is produced, end of story. But this is not always found. In snapdragons and fouro-clocks, crosses of white with crimson produce pink progeny. Interbreeding heterozygous F1 pink progeny produces white, pink, and crimson in the expected 1:2:1 ratios in the F2. In these cases the crimson allele is "incompletely dominant" over the white allele in Mendelian terms. Today we can explain it as the homozygous crimsons have two genes producing twice as much enzyme, more color, than the heterozygotes.
We have evidence the genes for pH work this way. It's reported petunias have as many as six genes that affect petal pH. Each acts independently and the final effect is a combination of distinct alleles acting. Remember, this is not like mixing paint! There would be 64 combinations, 64 hues if six genes were acting and each allele had equivalent effect. That's a lot, but still representing discrete differences, not a continuum. We don't know how many genes rhodies have. It's not unreasonable to suggest rhodies have "several." We'll only discover those in which there is variation in the alleles. We also don't know if each allele contributes the same increment of pH change, but that certainly must be the initial hypothesis. Okay, we have questions. Let's find some answers! A scientist would use a pH meter for measurement, but in my experience our eyes are exquisitely adapted for making such fine distinctions and divisions - better than any meter. I selfed deep purplish red 'Dan's Early Purple' and got several repetitions of distinct hues from vivid purplish-red to vivid reddish-purple. We should expect there to be distinct differences. How many distinct hues can we find between bright red and our bluest purple? They wouldn't necessarily have to be related (though a good cross might reduce the variables). Could we do it at a show?
Half a Half-dozen
In case it wasn't dwelt upon enough to become clear, we're speaking of these enzymes in a functional sense. When we speak of flowering plants all sharing genes that specify enzymes like dihydroflavonol 4-reductase, DFR for short, we're not saying all plants make exactly the same molecule. Orders, families, genera, and species have inherited mutations or sports and passed them on, perhaps "sported" again. The enzymes all share identifiably similar structures, nearly the same sequences and are meant to perform the same function, even if some plants may have "broken" versions.
How does this inform us as we look around the garden? Certainly one of our goals is a true-blue rhody. Frankly, there aren't any. We've plenty of purples, some bluish. Malvidin and petunidin are purple pigments. Malvidin and petunidin are derivatives of delphinidin. But as we've seen, it is common for mutations to make nonfunctional enzymes, breaking synthesis pathways. Thus if our purples were produced by either petunidin or malvidin, we expect to see mutations of 5'MT and 3'MT breaking the synthesis of these purples, therefore, along with natural variations in pH, true delphinidin blues. So we see no evidence of delphinidin synthesis, i.e., an allele to make functional F3'5'H, in rhodies, and must conclude three of the six are missing. That chameleon cyanidin, with its range from red to blue depending on pH, seems to explain the color of most rhodies. When cyanidin is produced, peonidin becomes a possibility. Rose breeders prefer peonidin. It doesn't tend to "blue" as blooms age. So perhaps our really good reds, R. barbatum , strigillosum , haematodes , 'Taurus', or 'Haldfan Lem' use peonidin. Finally, that leaves us to discover if red-orange pelargonidin is present in rhodies, perhaps in azaleas. Knowing how pigments are produced, and observation informs us we can only expect three fundamental pigments, modified by pH, in our red, purple, and bluish rhodies.
DFR is believed to be "picky" about its use of "raw materials." It appears elepidotes (subgenus Hymenanthes ) have a DFR that only works with dihydroquercetin, leading to cyanidin. In azaleas we see pigments that appear to be truly golden oranges through deep brick red 'Gibraltar', not appearing to be blends of red and yellow (though carotenoids are reported in subgenus Pentanthera ). I suspect in some species of subgenus Pentanthera a slightly different DFR uses dihydrokaempferol, leading to pelargonidin synthesis. Could a rhody have alleles for both DFR enzymes, one using dihydrokaempferol and the other dihydroquercetin made by F3'H, thus producing both cyanidin and pelargonidin? If we're assuming pelargonidin synthesis, then yes, certainly. Paper chromatography, another high-school science-fair-level project, would resolve this issue, as well as cyanidin and peonidin in reds. (Did you know common coffee filters could be used?)
Intensity: Theme and Variations
We have been much concerned with producing pigment molecules, rightly so. But colorless flavonoids produced in associated synthesis pathways have been seen to play important roles in intensity. They are called "co-pigments." On their own they are not yet studied as much as the pigmented molecules. They just keep turning up in those studies.
Once flowers produce the half-dozen characteristic anthocyanin pigments, other enzymes may add "decorations," as some of the literature calls them, around the edges. That can modify the hue to some extent but doesn't throw it open to infinite variety. However, they do seem to contribute greatly to intensity and pigment stability.
The "flavylium" substructure is shared by the flavonoids and anthocyanins. They have the same size and shape. This means they can be "stacked" nicely. Flowering plants have contrived to do exactly that. In some cases the "decorations" mentioned above help hold things in place. Examples have been found in which virtually any of the hydroxyl (‑OH) groups have been methylated (‑OCH3), such as the synthesis of peonidin from cyanidin or petunidin and malvidin from delphinidin. Some plants use a molecular bridge between two flavylium parts that holds them together. Stacking allows the electrons, represented by the straight lines and large circles, in one molecule to influence similarly situated electrons in an adjoining molecule (see Fig. 1). That affects the molecule's predilection for absorbing light. Co-pigments have this shape too. They can also participate in this "Dagwood sandwich" stacking of pigment molecules with co-pigments. Stacking our anthocyanins with flavonoid co-pigments increases the chances that a photon of light impinging on the complex will be absorbed, hence increasing intensity (although pH also has an effect on intensity). Cornflowers use 3 cyanidin molecules with 3 co-pigment molecules, held together by a few metal ions. It's quite apparent that pigment stability is an issue in certain families of rhodies, "yak-ish" hybrids in particular. This also has a molecular basis. Anthocyanins aren't particularly stable on their own. They derive much of their stability from these “decorations” and complexes. So the "faders" seem to have all the enzymes we've identified that produce anthocyanins (pigmented in bud) but, through the same sort of genetic mutation, lack functional enzymes to do the additional "decoration." Can we see other evidence in the garden? Have you ever observed that while we have bright yellows and light yellows, yellows don't seem to fade badly? Yellow elepidotes have the flavonoid anthoxanthin pigments, so this seems to show fading is peculiar to anthocyanins rather than something destructive of pigmentation in flowers. Neither do carotenoids fade.
In the question "which is the gene for white," we saw that a mutation in any of these genes producing a "broken" enzyme leads to loss of color. Thus the answer is "they all are." Crosses between whites with different, complementary non-functional enzymes can allow color to reappear. Similarly, crosses of two complementary pinks, for example, could produce reds.
Co-pigments and decorated anthocyanins are made and function in the same general ways already presented. Genes specify enzymes that use precursor molecules created by other enzymes in a chain of syntheses. It will be a long time before rhodies are characterized enough that we can look these up in some reference, so we should be prepared to fit our observations into this scheme found in nature. Besides, individual plants may not care what the books say.
Before we leave the topic, these genes and enzymes that "decorate" anthocyanin and other flavonoid molecules and stack them into intensely colored "sandwiches" are to some extent independent of the colors they intensify. The difference between an intense red and an intense purple is primarily pH 2 . They're more alike than different. 'Borde Hill' and 'Black Magic' are deeply colored reds. There's something going on with the cyanidin (or peonidin), but that wouldn't necessarily work on flavonoid yellows. We shouldn't expect that. The genes that lead to intensity need the proper initial molecules. But those genes probably could intensify the pink of a "yak." For example, 'Fred Peste' (( R. degronianum ssp. yakushimanum x 'Corona' [a pink]) X R. haematodes ) has the strong color of its pollen parent. The seed parent already had what was needed for red, enzymes in the cyanidin pathway; what it needed was the intensity brought by R. haematodes .
Within petal cells, anthocyanins and flavonoids are deposited in vacuoles. So extraneous genetic factors affecting the production and distribution of vacuoles can also appear to affect pigment intensity. Carotenes wouldn't be in these vacuoles.
Absorbed
Depending on how it is observed, light might exhibit properties of a ray of particles, or an oscillating wave, though it is truly neither. Electrons usually seem to be particles, but not always or entirely, hence "electron microscopes." It gets interesting when light of a certain frequency wiggles in the vicinity of an electron in an atom or molecule. Then the electron might absorb the photon of light, changing its energy level. In atoms the frequency of the light absorption, and its re-emission, are very precise - spectra show sharp lines. The light of mercury or sodium vapor lamps, not to mention lasers, demonstrates this. But in molecules like our pigments, the electrons absorbing light are those connecting the atoms, especially those represented by the large circles in the illustration, rather than electrons in the thrall of an atom. Molecular absorption is spread out, more or less broadly. For example, chlorophyll absorbs most of the light in the red-yellow and blue-violet portions of the visible spectrum of white light, leaving the green to be reflected. Cyanidin in low pH, which produces a red rose or rhody, absorbs violet through yellow from the spectrum. (In the red light of a photographer's darkroom, with no yellow to violet light to absorb, it looks as a white rose would.) Higher pH shifts this absorption band to higher frequencies.
Clearly, a mixture of two pigments combines the absorption spectra of both. Sometimes that can be used to advantage, sometimes it must be. But sometimes it's best avoided. Orange has it's own, albeit narrow, place in the visible spectrum. The good, clear orange of potassium dichromate absorbs red and violet through yellow, but trying to make orange by mixing red and yellow isn't the same. The red pigment absorbs violet through yellow, and the yellow pigment absorbs red and violet through green.
On & Off
It's generally known that every organism has the entire genome in virtually every cell. However, that doesn't mean that all the genes are active all the time. Some are active only periodically, e.g., those involved with seasonal flowering, or with cell division. Consider the production of pigment we've been exploring. Is there any reason for those genes to be active after the flowers are all produced? It does take energy to produce pigment. It would be to the plant's advantage to switch them off when they aren't needed to color flower petals and attract bees. Sometimes genes will be "switched off" permanently - the fundamental fact behind stem cell research.
How do we see this in the garden? I'm sure you have. How about picoteed blossoms? As a picotee rhody bud begins to develop all the genes are functioning, making enzymes that will synthesize pigment. Color is deposited in the petals as they grow out. Now, through some "sport" in gene regulation, one gene in this chain is switched off before petal growth is completed. One enzyme required for pigment synthesis, perhaps CHI, is no longer produced. (To say enzymes are catalysts and not consumed by the biochemical reactions they mediate is not to say that once produced they are active forever.) So the blossom has a ring of color on its lips, but a colorless throat. In the natural world this could be a disaster, because bees see the UV absorption of anthocyanins. But humans find the color pattern fetching. Notice that if the affected enzyme were F3'H then yellow flavonoids could still be produced. Now we get red/pink and yellow bicolors so common in R. dichroanthum hybrid descendants. Observe this closely: what was pink becomes yellow, without a satisfying orange at the boundary.
On the other hand, what if the gene was initially off and switched on late? Then we get colored throats and eyes. One gets the impression of a rather distracted plant that forgets what it should be doing. Another example of genes turning on and off are the spots, e.g., those in 'Spatter Paint'. Blotches seem to be an extension of spotting. It seems the genes responsible for pigment synthesis enzymes within the spots and blotches are different from the rest of the petal. Pink or red (anthocyanin) petals with yellow (flavonoid) spots and blotches aren't unusual. Looks like F3'H is involved again. Green spots are even seen. Could that be chlorophyll? That's interesting.
Couldn't pigment enzyme genes being switched-off permanently cause whites? Possibly, I suppose. Loss of pigmentation, white, is common in organisms - cats and rats, roses and roosters - though pure whites, pure white in bud and blossom, seem surprisingly rare in rhodies. Most "white" rhodies seem to have some color synthesis, ineffectual as it may be. Much genetic research over the past century has shown us how gene mutations affect the enzyme systems. Malfunctioning enzymes are a direct result of mutation. Examples of point mutations causing amino acid substitutions in proteins have been found generally. 3
What makes 'Yaku Fairy', a 20-year- old tuffet two feet across and a foot high, so different from R. decorum ssp. diaprepes that can pop a new branch that grows a foot or two in a summer? It's the same fundamental process as demonstrated here for color. When we look at 'Yaku Fairy', we should think of Figure 1 (Part I) and the way a malfunctioning CHI can stop the entire chain of color synthesis. But this time a malfunctioning enzyme early in the synthesis chain for growth factors, instead of color, stunts growth. Middling-sized plants synthesize more of the chain, and in diaprepes the synthesis goes all the way. As I wrote early in the prior article, while this has been primarily about color, because it's easier to see, the processes are essentially the same for all characteristics we hybridize. It underlies all our interpretations of what's going on. The process presented here is universal.
Orange
You may have noted I've touched on orange in various ways. If not a true "delphinium blue," elepidote breeders with their yellow and pink bicolors and blends may covet the really bright orangey colors of RR. calendulaceum , austrinum , or flammeum and the Exbury azaleas more than anything. Elepidote hybridizers have tried to create orange by combining red and yellow. If the yellow were a carotenoid, produced by an independent terpene synthesis chain, that would be easy. Indeed, look at the yellow admixture in Hemerocallis ; it could be hard to get out!
I expect 'David' X 'Crest' was done for this reason. Red 'David' X yellow 'Crest' makes reds with no trace of yellow (as seen of JE #256, #487 in the TVARS garden at Jenkins Estate, Aloha, Oregon). 'David' provides genes and enzymes that use the precursors of those yellow flavonoids as precursors to synthesize red cyanidin. The same effect is seen in pink 'Airy Fairy' (yellow R. lutescens X pink R. mucronulatum 'Cornell Pink'). But, "you can't have your cake and eat it too," not an orange.
An inefficient F3'H might be thought to do the trick. Some of the dihydrokaempferol would be converted to eriodictyol and dihydroquercetin leading to some cyanidin, pink in a low-pH petal, leaving some to be converted to yellow flavones and flavonols by FNS and FLS. Theoretically, this way one might produce a blend of yellow and pink, appearing orange. But either way, it seems to have been very hard to do. Now we know why. Not only is this problematic from the way anthocyanins and other flavonoids are synthesized, but think of the combined absorption spectra. Even when something more or less orangey is produced this way, to my eye it lacks the brilliance of the Exbury's. I'm sorry, but getting an impression of orange thirty yards away from a yellow and pink bicolor just doesn't do it for me, even in my seedlings (I can see one out my window as I write this).
If we want true orange in our elepidotes, it would seem we should hunt for fertile azaleodendron hybrids. We could try to get the DFR that can use naringenin from subgenus Pentanthera to make pelargonidin. Or perhaps we could get carotenoids from them. Tetraploid R. calendulaceum would be a poor choice for diploid subgenus Hymenanthes elepidotes, but perhaps R. flammeum or R. austrinum can bring us this allele. (I didn't promise this would be easy!)
Getting from There to Here
In 1859 Charles Darwin published The Origin of Species and then only because Alfred Russell Wallace had come to the same conclusions and was about to publish his own work. Few scientists have been so abused, or had their work so abused. Darwin expected that. He's thought to have hoped to publish posthumously.
In those days nobody truly understood heredity. Darwin's key insight was that all species produced more progeny than their environment could support, and natural (genetic) variations gave some individuals survival advantages which were propagated in succeeding generations. "Natural selection" is the common term, but selection doesn't have to be "natural." Since the dawn of the agricultural age 10,000 years ago, selection is how we have forced adaptation of wild species to domestic use. (Don't get hung-up on some Rousseauian concept of what's "natural.” My environment has curiously shaped pieces of glass in it, so I am not at a survival disadvantage at 65MPH or from a baby rattlesnake coiled in the dust.)
In 1866 Augustinian monk Gregor Mendel published his studies of inheritance in peas in an obscure Bohemian journal. It remained undiscovered until 1900, when Correns, DeVries, and Tschermak independently and individually reproduced his results. Reproducibility is critical in science.
Mendel's key insights were: 1) using pure-breeding varieties that differed in just one trait, 2) quantifying his results. Mendel established that inheritance of traits was a discrete and predictable process. He showed that inherited traits segregate independently. As we will see, he got lucky about that.
We should realize we should be using both Darwin and Mendel in our hybridizing. Mendel when we're pollinating, but Darwin for the sweep of our projects.
After Mendel's work was rediscovered, it didn't take long for cytologists to realize little rod-like things they could see in stained cells during the process of division, now called chromosomes, behaved in a way that could explain Mendel's model. But they had no proof! Optics got better over the subsequent years, as did staining methods, and more genetic characteristics were discovered. Then the "Lords of the Flies" in Thomas Hunt Morgan's lab discovered one of these characteristics was the result of a missing dark-stained band, a deletion, visible on a chromosome. Bingo! Following that came Watson and Crick, equally important Franklin and Wilkins, and the discovery that strands of DNA are the essential parts of chromosomes, but we're not going that direction.
One of the first realizations of genetics occurred as a consequence of a chain of discoveries. Mutations caused differences in genes, called alleles. Genes occupy specific places on chromosomes. Chromosomes are passed from parents to progeny as a whole. Most organisms have pairs of chromosomes, polyploid plants excluded. Therefore, for a given gene each pair of chromosomes might have matching alleles, called homozygosity, or different alleles, called heterozygosity. And that explained Mendel's results.
Mendel crossed tall and short peas, got all tall peas in the first generation, but got short peas back in the second generation. With tall purple peas and short white peas, he could make short purple peas on a whim. So Mendel tells us about heredity. It's important to realize the unitary nature of inheritance. The units of heredity, now called genes, are located on chromosomes. We can observe chromosomes and watch them participate in cell division and gamete production. The function of a chromosome is to transport genes. The function of a gene is to store information, the "parts list" for a protein. Enzymes are specific proteins that not only have a structure; they work because of that structure. Everything has a unitary structure - which is why it's not at all like mixing paint. Genes are passed through generations, yet retain their identity. Mutation changes them, not "association." That means we can plan, make crosses, and deliberately bring genes from different sources of chromosomes together in an individual with intention, even generations later.
Early in the 20th century when genes were discovered, and as the reproducibility of DNA was realized in mid-century, it was believed genes were "sovereign." Genes controlled organisms. But if genes were alone in their nuclear environment doing their thing, then a problem was realized: explaining how organisms could respond to their external environment. In the current paradigm, the genetic code specifies proteins that are then the primary "actors" in the organism, participating in chemical reactions influenced by such as temperature and energy absorbed light, e.g., photoperiod, and even "feedback" to influence gene activation.
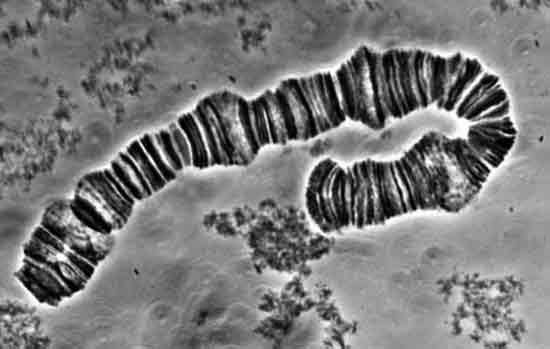
|
|
Fig. 2. Banding of a polytene salivary chromosome in a Varve midge
from the Connecticut River. Ed Klekowski, University of Massachusetts, Amherst. Used with permission. |
The Numbers Game
My presentation has been much rooted in genetics. One objection about mixing hybridizing and genetics is a belief that genetic analysis requires raising many (eventually discardable) seedlings until the characteristic of interest can be judged (3-5 years for flowering), so one can count and determine how the proportions follow Mendel's laws. But it doesn't have to be that way! Don't let that put you off. Every seedling we raise from a cross reveals something! Maybe it's something new, maybe not. But if we're not paying attention, we'll never learn the new secret.
When I was persuaded I could only raise rhodies from seed, the presence of 'Evening Glow' in my foundation planting, and preference for fragrant flowers, suggested a project making a fragrant yellow by back-crossing on something in its ancestral R. fortunei series. If one looks carefully, the throat of 'Evening Glow' is as bright yellow as we have in elepidotes. But I dislike the pinkish streaks inherited from R. dichroanthum . Virtually everything from R. dichroanthum seems to have this nasty blend of pink and yellow. My first question was whether the pink and yellow could be separated or were inextricably combined as part of what comes from R. dichroanthum . My first cross was to something of uncertain origin, but keys out to a member the R. decorum family, with a pale yellowish throat, pale pinkish lips, extra lobes and fragrance.
The first seedling that bloomed was a clear, bright yellow without a trace of pink and some fragrance, which promptly died. Still, I had my answer! A million seedlings would not have proved it was possible, but just one proved it was not impossible. I've since gotten bright, clear yellows by selfing 'Evening Glow', proving the point. It isn't necessary for me to raise hundreds. The modest number I do have tells me what I need to know about the genetics. I can carry on. It now seems the plethora of yellow-pink bicolors coming from R. dichroanthum is because that's what other hybridizers selected and registered, not something special in the genetics. I got those too, some with extravagant calyces - not my preference.

|
|
Fig. 3. Chromosomes duplicating and separating during mitosis
[Ed. note: probably allium root tip]. Eric J. Kunnen, Grand Rapids Community College. Used with permission. |
Now, to be sure, with my modest numbers in both crosses I may not have the brightest yellow I might have had or gotten the most fragrant one possible, but I am making progress. My goals are modest, only three characteristics: bright yellow, fragrance, and some hardiness. If one were more ambitious, and hoped to get some combination of several characteristics in just one cross, it could be a numbers game. Mathematically, combinatorics grow exponentially, but we can stay on the right side by dealing with no more than a few characteristics in each project, and perhaps approach a complex goal with projects from both sides.
Lucky Number 13
Now here's where it gets interesting for us. Rhodies have been observed to have 13 unique chromosomes. Logically, if rhodies had only 13 genes, rather than tens to hundreds of thousands, each could be on a separate chromosome. But with the 14th gene and more, perforce they must share chromosomes. Indeed, microscopic examination of chromosomes shows lots of those staining bands identified with genes. So all those thousands of genes must be distributed among thirteen "linkage groups." The seven characteristics Mendel reported on all came from different linkage groups, so they assorted independently.
Let's take a brief look at the consequences of genes sharing chromosomes. We can observe how paired chromosomes reproduce and separate independently in gametes to recombine in fertilization. Each seedling receives one of each from each parent. Consequently, each seedling inherits one chromosome with its complement of alleles of genes in this linkage group from one parent, and another with, especially if they're hybrids, a different complement of alleles of the same genes in this linkage group from the other parent. What happens when it becomes a parent? One chromosome or the other goes to each seedling, bringing with it one set of alleles or the other. That which was brought together is separated again. Hybridizers know it well.
But wait! What if what we want is to bring together two alleles from two genes in the linkage group from different parents onto one chromosome so we can have them passed on together: "breed true" as commonly said? Mother Nature has also discovered that this can be useful, so chromosome duplication is almost but not entirely perfect. In a process called "crossing over," the two strands of DNA can become severed and reconnected to their other partner. How often it is observed depends on how close those genes are to each other. It can be as much as 50 percent, giving the appearance that the genes aren't in the same linkage group at all, except when both are linked to a third gene that's in the middle! So if we want alleles from different parents linked together, it is possible - maybe easily, maybe not.
Remember the prevalence of colored seedlings from crosses among whites of R. catawbiense in the Bowers letter quoted by Craig? Not only does that provide us evidence of two different but complementary mutations, but also suggests they are in different linkage groups, on different chromosomes.
In my fragrant yellow project with 'Evening Glow', I can see that I have functional genes and enzymes to get as far as the yellow anthoxanthins. I've also got a bit of picotee in pink cyanidin, principally between the petal lobes. Restated properly, there is a functional gene for the F3'H enzyme leading to cyanidin production which is being allowed to function in parts of the petal for a while, but is then being "switched off" by another. There's reason to think it came from R. dichroanthum on one side of the family, and therefore heterozygous. What allele might be on the other chromosome? It appears to be a gene that makes nonfunctional F3'H - 'Evening Glow' isn't all red! My experiment to see if the pink and yellow could be "separated," was in reality to see if the functional F3'H gene was in any linkage group with the other genes I needed for just clear yellow and fragrance. If it were in the same linkage group, then they'd "go together" until crossing over separated them with whatever frequency. As it is, it appears they're on different chromosomes. Amazing! One cross and I've got a bit of the picture about which genes are on different chromosomes, or at least far enough from each other that they might as well be. One lonely data point, but this is how genetic maps come to be.
It will be many years before we have useful maps of the rhody genome. Don't wait! Mendel's results came from a monastery garden, not a science lab. As we get results from a cross, those do show what the genes are doing. Begin to analyze which hypotheses explain them and discard those that don't. That's what the geneticists do, we can too! We may have a few possibilities to work with, but that's better than a total mystery. Limited as those may be, ignorance isn't better!
It may be fun to see the unexpected results from some of our hybrids. I wonder if 'Peeping Tom' was expected when R. wardii and 'Mrs. Furnivall' were crossed. When we say "that's odd" we ought to prick up our ears, rather than shrug and move on. I hope to have demonstrated that these unexpected results can often be very revealing if we can just puzzle them out. Anyone wanting to use a R. dichroanthum descendant for yellow but not wanting the added pink appearing in most of the registered ones, persevere! Those genes will come apart. I saw it happen. When Mother Nature reveals a secret to us, we ought to be prepared to observe it! Mother Nature has always been whispering to us. She has amazing tales to tell. We have to learn her language.
References
Rogers, Paul. 2008. Why is it pink, Daddy?, Journ. Amer. Rhod. Soc. 62:2:91-96.
Greer, Harold E. 1996. Greer's Guidebook To Available Rhododendrons, Species & Hybrids , third edition. Eugene, OR: Offshoot Publications.
Jones, Jeff R., Thomas G. Ranney, Nathan P. Lynch and Stephen L. Krebs. 2007. Ploidy levels and relative genome sizes of diverse species, hybrids, and cultivars of Rhododendron . Journ. Amer. Rhod. Soc. 61:4:220-227.
Craig, Donald. 2006. Nothing new under the sun. Journ. Amer. Rhod. Soc. 60:1:30-34
Further Reading
Griesbach, R.F. 1987. Rhododendron flower color - genetic and cultural interactions. Journ. Amer. Rhod. Soc. 41:1:20-21.
Footnotes
1 Exceptions: some animals "steal" toxins from what they eat and use them for defense. Nevertheless, they were synthesized in their original species.
2 Until we have concrete evidence of functional F3'5'H, hence delphinidin and the possibility of typically purple malvidin and petunidin, we should assume cyanidin. As carotenoids are yellow, but not all yellows are carotenoids, so malvidins may be purples, but not all purples are malvidins. Cyanidin even makes a respectable blue in Mecanopsis betonicifolia .
3 With the discovery of DNA, what we learned since Mendel was wrapped-up in a simple, elegant package. Gene regulation, epigenetics, methylation, preservation of "junk DNA", introns, exons and transposons have been characterized relatively recently and are not entirely as well understood yet. It reminds me of post-Newtonian physics. For rhody hybridizing, let's understand the basics first!
Paul Rogers, BS Chemistry, 1967, California State College - Long Beach, has been seriously raising animals, which must be bred and cannot be "vegitatively propagated," since he was 8 years old, using genetics since a high school student. He made the "mistake" of trying to identify some rhodies at a new home, and came under the influence of TVARS members. His main "project" is the production of a fragrant bright yellow.
