JARS v38n2 - Micropropagation of Rhododendrons
Micropropagation of Rhododendrons
Steve McCulloch, Olympia, WA
Steve McCulloch is the Micropropagation Manager at Briggs Nursery, Inc. in Olympia, Washington. He studied under Dr. Brent McCown at the University of Wisconsin, where he earned his B.S. and M.S. degrees in Horticulture.
For the last six years or so, rhododendron cultivars and species have been propagated using a relatively new technique called micropropagation. Micropropagation is the propagation of plants on an artificial medium under aseptic (germ-free) conditions from very small pieces of plant.

|
|
Rhododendron 'Dorothy Amateis'
a selection now micropropagated. |
The history of micropropagation, sometimes referred to as tissue culture, is fascinating. Doing research in 1902, Gottlieb Haberlandt is credited with being the first to attempt isolation and culture of plant tissue with aseptic conditions. Although unsuccessful, this plant physiologist set the stage for further research. Through the efforts of several individuals and much research, proper media, growth regulators, equipment and techniques were developed to grow and proliferate several different plants.
|
|
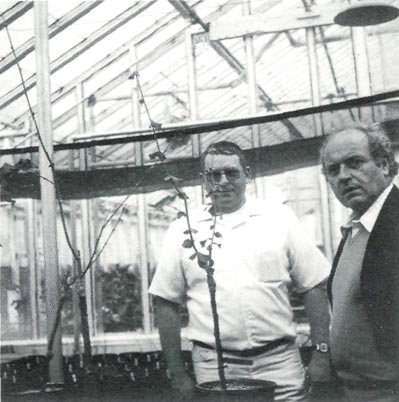
Fig. 2 Dr. Wilbur Anderson (center), pioneer in
researching the micropropagation of Rhododendron. |
In 1968, Dr. Wilbur Anderson began research on micropropagation of Rhododendron . (Fig. 2) Working at the Northwestern Washington Research and Extension Center in Mt. Vernon, Washington, Anderson was able to develop a low salt medium with appropriate growth regulators to support the growth and multiplication of Rhododendron 'Rose Elf' shoots. Through the efforts of a number of individuals and organizations, including the American Rhododendron Society, enough funding and backing was secured to support this research.
Micropropagation is certainly not the only way to propagate rhododendrons. The growing of seed, layers, cuttings and grafts are still very good techniques. Then why use micropropagation? This technique offers the propagator, rhododendron breeder and consumer the ability to:
1. Propagate plants considered difficult or impossible to propagate.
2. Introduce new plant material more rapidly.
3. Maintain and produce pathogen-free plant material.
4. Propagate plants year round.
5. Eliminate the costly maintenance of stock plants.
6. Produce a dependable supply of plants every year.
Most plant shoots can be grown on a gelled, agar medium containing water, sugar, vitamins, growth regulators and macro- and microelements. Several formulations are used. Most woody ornamentals require or grow best on a medium with a low concentration of mineral salts. The inorganic salts and other components are combined with deionized or distilled water and the pH is adjusted to 4.5. Sucrose (table sugar) and agar (gelling agent) are added to the solution and it is heated. (Fig. 3) This hot mixture is then poured into glass test tubes or jars and capped. The medium is sterilized by autoclaving at 121 °C (250° F) and 1.1 kg/cm 2 (15 lbs/in 2 ) for 15-20 minutes.

|
|
Fig. 3 Culture media being
heated on the stove. |
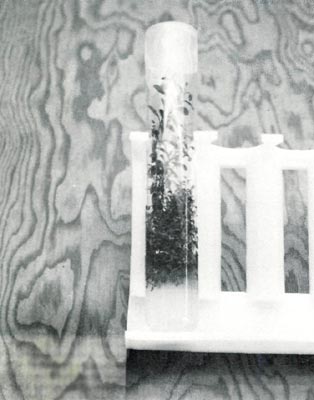
For multiplication of shoots, cytokinins are added to the medium. These chemicals when included in the medium promote axillary bud formation and encourage branching. Think of it as chemical pruning without having to remove the apex. When a single shoot is placed on the proper medium, most of the axillary buds will expand and form new shoots. But since these new shoots also have vegetative buds along their stems, they will also expand to form new shoots. This process repeats until some factor is limiting. (Fig. 7) This means of multiplication can be used to increase the number of shoots of a rhododendron.
|
|
Fig. 7 A multiplying culture of
Rhododendron 'PJM'. |
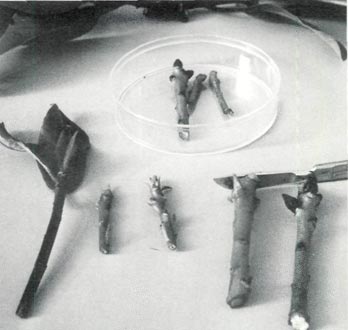
The micropropagation of rhododendrons begins with the excision and decontamination of a shoot and placing that un-rooted cutting on a sterilized artificial medium. (Fig. 4) Green wood shoot tips 4-6 cm are used in starting rhododendrons. Cuttings with the leaves stripped off are placed into dilute laundry bleach and agitated (Fig. 5). Usually 15-25 minutes is sufficient to kill surface bacteria and fungi. Contaminants, if present, will appear within 10 days.
|

|
|
| Fig. 4 Shoot tips prepared for decontamination. | Fig. 5 Agitation by vigorous shaking. |
Once decontaminated and placed on the proper growth medium, buds expand and form new shoots. With time and some luck, these shoots will become more juvenile or seedling-like in shape and start to multiply. There is a physiological change in the plant when cultured. Since juvenile rhododendrons root readily, this can be used to our advantage in propagating hard-to-root plants.
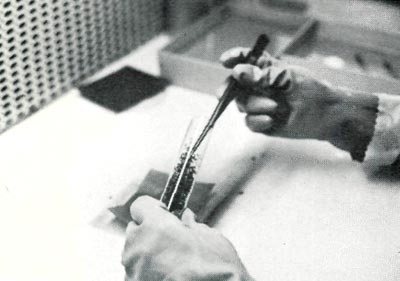
The opening and closing of sterile, autoclaved test tubes and jars, and the cutting-up of aseptic plants should be done in the sterile air of a laminar flow hood (Fig. 6). These hoods effectively filter out all aerial mold and bacterial contaminants. All instruments used to cut and transfer shoots need to be sterile. Workers need to be aware of sources of contaminants and ways of preventing the spread of them to clean cultures.
|
| Fig. 6 Transferring plantlets in a laminar flow hood. |
After the shoots are transferred to fresh media, they are placed in an illuminated room with fluorescent fixtures for four to ten weeks (Fig. 8). The room temperature should be kept about 24°C (75°F).

|
| Fig. 8 A culture room filled with several thousand plants. |
Juvenile rhododendron shoots are generally easy to root. Shoots may be rooted in the laboratory on a rooting medium or in soil. Shoots are stuck into soil and then placed in a high humidity environment. Good rooting usually takes between four to eight weeks. Once the plants are established, the humidity is gradually lowered and the plants hardened off.
Rhododendrons produced using micropropagation have performed well. Young cuttings of the harder-to-root selections grow vigorously due to a uniform root ball. Although we have had difficulty in culturing indumented rhododendrons, several will be available in the near future. Because of micropropagation, several new and exciting hybrids will be introduced faster to the commercial market for growers and hobbyists to appreciate and enjoy.
Further reading
Dodds, John H. and Lorin W. Roberts 1982.
Experiments in Plant Tissue Culture
. Cambridge University Press. Cambridge, England. 178p.
Kyte, Lydianne and Bruce Briggs 1979. A simplified entry into tissue culture production of rhododendrons. Proc. Inter. Plant Prop. Soc. 29:90-95.
Kyte, Lydianne 1983. Plants from test tubes - An introduction to micropropagation. Timber Press. Beaverton, Oregon. 132p.
Thorpe, Trevor A. (editor) 1981. Plant tissue culture - Methods and Applications in Agriculture. Academic Press, Inc. New York, NY. 379p.
Wetherell, D.F. 1982. Introduction to in vitro propagation . Avery Pub. Group, Inc. Wayne, NJ. 87p.
