JARS 43n2 - Micropropagation Of Flame Azalea
Micropropagation Of Flame Azalea
Frank A. Blazich and Juan R. Acedo
North Carolina State University
Raleigh, North Carolina
Reprinted from Journal of Environmental Horticulture
Abstract
Shoots tips excised from an actively growing stock plant of Flame azalea [
Rhododendron calendulaceum
(Michx.) Torr.] were surface sterilized, the terminal portions were removed (decapitated) and the shoots placed horizontally on agar-solidified Woody Plant Medium (WPM) supplemented with 15 ppm 6-(γ, γ-dimethylallylamino)-purine (2iP).Within 4 to 6 months multiple shoot formation commenced. After 2 to 3 additional months of growth, axillary shoots were excised from the original explants. The shoots were decapitated and placed on WPM. After 2 subcultures, 8-node axillary shoots were excised, decapitated and cultured on agar-solidified WPM supplemented with 0, 4, 8, 12, 16, 24, and 32 ppm 2iP. The greatest number of shoots (micro cuttings) > 5 mm (0.2 in) were produced at 12 ppm 2iP. micro cuttings >10 mm (0.4 in) were rooted using ex
vitro
procedures. Enhancement of both axillary shoot multiplication and shoot length was achieved by addition to the medium of 80 ppm adenine sulfate and 200 ppm NaH
2
PO
4
.
Introduction
Flame azalea [
Rhododendron calendulaceum
(Michx.) Torr.] occurs naturally in the Appalachian region of the United States, extending from southwestern Pennsylvania and Ohio to northern Georgia (7, 8). It blooms in late spring and is regarded as one of the most striking, native flowering shrubs with flower color ranging from orange-yellow to scarlet (8). The tremendous floral display coupled with the diversity of flower color provides incentives for selection and propagation of superior forms. However, before the horticultural virtues of this species can be exploited, techniques must be developed for vegetative propagation of desirable clones. One of the main reasons why this plant is not widely utilized is due to the lack of suitable procedures for asexual propagation.
One technique for vegetative propagation might involve the use of rooting stem cuttings. This would seem like a logical approach because stem cuttings of many Rhododendron species can be rooted without much difficulty. Unfortunately, stem cuttings of Flame azalea, particularly those taken from plants in the adult growth phase, are extremely difficult to root (13, 14). Pronounced clonal variation in the rooting response also places severe limitations on cuttings as a means to propagate selected clones(12, 14).
Difficulties associated with cloning Flame azalea by rooting stem cuttings might be overcome by application of tissue culture procedures which have been successfully used on many species of Rhododendron (1, 2, 3, 4, 5, 6, 10, 11). Therefore, the following study was undertaken to investigate the feasibility of propagating Flame azalea by micropropagation.
Materials and Methods
Experiment 1: Development of a workable procedure:
Terminal shoots, 2 to 4 cm (0.8 to 1.6 in) in length were taken from an actively growing, containerized stock plant maintained outdoors under lath shade in Raleigh, NC. The stock plant was in the adult growth phase and had been propagated sexually. Following excision, large leaves were removed. Shoots tips were placed in running tap water for 15 minutes, washed in soapy water for 1 minute and rinsed under running tap water for 10 minutes. Explants were then submersed for 15 minutes with gentle agitation in a 10% Clorox solution (1.05% sodium hypochlorite) containing 0.05% Tween-20, rinsed 3 times in sterile distilled water and the terminal portions and basal 2 to 3 mm (0.08 to 0.12 in) of each shoot were removed.
Shoot tips were placed horizontally into 100 x 25 mm (3.9 x 1.0 in) plastic Petri dishes containing 25 ml of Woody Plant Medium [WPM (9)] solidified with 0.7% agar, and containing 15 ppm 2iP (pH 5.2). Dishes were sealed with Parafilm® and cultures were maintained at 25° ± 1° C (77.0 ± 1.8° F) under a 16 hr photoperiod supplied by 40 W cool-white fluorescent lamps. The lamps provided a photosynthetic photon flux density (PPFD) of photosynthetic active radiation (PAR) between 400 and 700 nm of 56 to 80 µmol/m 2 /s (4.3 to 6.0 klx) plus a radiant flux density of photo-morphogenic radiation (PR) between 700 and 850 nm of 11 to 15.8 W/m 2 , measured at the shelf surface on which the dishes were placed. These and all other light measurements were recorded with a LI-COR LI 185A quantum/radiometer/photometer.
Shoots tips were transferred at 7 and 14 days to fresh medium and subsequently every 2 to 3 weeks until multiple shoot formation commenced which took 4 to 6 months. Following multiple shoot formation, explants were transferred to 80 x 100 mm (3.1 to 3.9 in) Pyrex storage jars containing 100 ml of the same medium on which the explants were initially cultured. Within 2 to 3 months, axillary shoots > 1.0 cm (0.4 in) were excised from the original explants. The terminal portions were removed (decapitated) and the shoots placed horizontally into similar Pyrex storage jars containing the same volume of WPM (9) as described previously. After 2 subcultures,. 8-node axillary shoots were decapitated and placed horizontally in 100 x 25 mm (3.9 x 1.0 in) plastic Petri dishes containing 25 ml of WPM (9) supplemented with the following concentrations of 2iP: 0, 4, 8, 12, 16, 24 and 32 ppm. Each concentration was replicated 5 times and a replication consisted of a Petri dish containing 5 decapitated axillary shoots. Cultures were placed in a Percival MB-54 growth chamber maintained at 25° ± 1° C (77° ± 1.8° F) with a 16-hr photoperiod supplied by 40 W cool-white fluorescent lamps. The lamps provided a PPFD of 31 to 43 µmol/m 2 /s (2.3 to 3.3 klx) plus PR of 6.3 to 8.9 W/m 2 as measured at the shelf surface on which the dishes were placed. After 4 weeks, shoots were transferred to 80 x 100 mm (3.1 x 3.9 in) Pyrex storage jars containing 100 ml of fresh medium and left for an additional 16 weeks prior to data collection which included the number and length of all shoots (micro cuttings) > 5 mm (0.2 in).
Micro cuttings > 10 mm (0.4 in) were rooted under high humidity conditions in plastic flats [21.6 x 16.5 x 5.1 cm (8.5 x 6.5 x 2.0 in)] containing a sterile rooting medium of 1 peat: 1 vermiculite (by vol.). High humidity was provided by daily syringing of the micro cuttings with water and enclosure of the flats in sealed plastic bags. The cuttings were maintained for rooting under conditions virtually identical to those in the culture room. Percent rooting was noted after 10 to 12 weeks followed by acclimation of the rooted micro cuttings to reduced humidity and finally acclimation to greenhouse conditions and observance of subsequent growth.
|
|---|
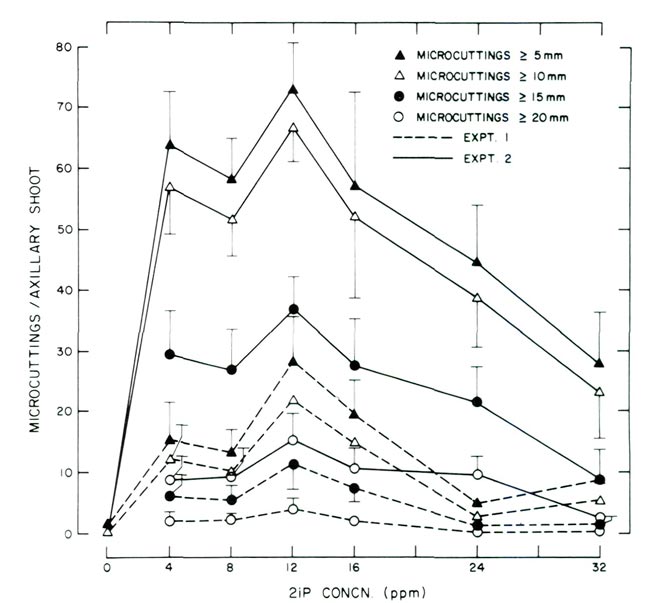
Figure 1. Response of decapitated, 8-node axillary shoots cultured on 2 media supplemented with varying concentrations of 2iP. Experiment 1 utilized WPM (9) and experiment 2 utilized WPM (9) modified with the addition of 80 ppm adenine sulfate and 200 ppm NaH 2 PO 4 . Data points for experiments 1 and 2 are based on 25 and 20 observations, respectively. Vertical lines represent ± 1 standard error and were omitted when < 1.0. |
Experiment 2: Enhancement of both axillary shoot multiplication and shoot length: From cultures maintained in the culture room as described for Experiment 1, 8-node axillary shoots were excised. The shoots were decapitated and placed horizontally in 100 x 25 mm (3.9 x 1.0 in) plastic Petri dishes containing 25 ml of WPM (9) (pH 5.2) solidified with 0.7% agar and supplemented with 80 ppm adenine sulfate, 200 ppm NaH 2 PO 4 and the following concentrations of 2iP: 0, 4, 8, 12, 16, 24 and 32 ppm. Each concentration was replicated 4 times and a replication consisted of a Petri dish containing 5 decapitated axillary shoots. Cultures were placed in the Percival growth chamber as in Experiment 1. After 4 weeks, shoots were transferred to 80 x 100 mm (3.1 x 3.9 in) Pyrex storage jars containing 100 ml of fresh medium and left for an additional 16 weeks prior to data collection which included the number and length of all micro cuttings > 5 mm (0.2 in).
Results and Discussion
Consistent with reports for other
Rhododendron
species (1, 2, 3, 4, 5, 6, 10, 11), Flame azalea can be propagated by tissue culture. Multiplication of axillary shoots was achieved by varying the 2iP concentration in the basal medium. The number and length of the shoots was enhanced by adding adenine sulfate and NaH
2
PO
4
to the medium (Fig. 1). Rooting and subsequent acclimation of tissue culture produced micro cuttings ranged from 70 to 90% whether originating from Experiment 1 or 2.
Regardless of the 2 media utilized, the greatest number of micro cuttings > 5 mm (0.2 in) was produced with 12 ppm 2iP. The same relationship was noted for micro cuttings > 10, 15 and 20 mm (0.4, 0.6 and 0.8 in). At concentrations < or > 12 ppm, the number of micro cuttings in each length classification decreased.
Although all micro cuttings > 5 mm (0.2 in) were recorded, the investigators feel that in order to effectively utilize ex vitro methods for rooting, micro cuttings must be at least > 10 mm (0.4 in); an ideal length would be 20 to 30 mm (0.8 to 1.2 in). In fact, the desire to increase the length of micro cuttings is what prompted Experiment 2. Despite Experiment 1 providing micro cuttings which could be rooted using ex vitro procedures, it was felt that the length of cuttings > 15 mm (0.6 in) needed to be increased. For example, the number of micro cuttings produced in Experiment 1 at 12 ppm 2iP > 15 and 20 mm (0.6 and 0.8 in) was approximately 11 and 4, respectively. Addition of adenine sulfate and NaH 2 PO 4 increased the number of cuttings > 15 and 20 mm (0.6 and 0.8 in) to approximately 37 and 15, respectively.
Micro cuttings of Flame azalea were relatively easy to root. Provided desiccation was avoided, percent rooting often approached 100% with the percentage of greenhouse acclimated plants always being less. Although micro cuttings rooted without difficulty, growth following rooting presented problems. Plants tended to grow as single stems whether or not they were decapitated or cut back more severely. This same phenomenon has been observed by the authors and reported by nurserymen when the species is propagated by seed. Seedlings tend to exhibit little or no branching and various types of pruning often do not alleviate the problem. Further study will be necessary to increase branching for plants propagated both by seed and tissue culture.
A critical step in developing the micropropagation procedures described was successful sterilization of actively-growing shoot tips taken from the stock plant. Initially 20 shoot tips were placed in culture (1 shoot/ culture) and within a month all but 4 were lost due to contamination. Contamination may have been lessened if the stock plant had been maintained for some time under greenhouse conditions before excision of shoot tips. In any event, experience has shown that failure to properly sterilize shoot tips can present problems.
The successful protocol described for micropropagation of Flame azalea is very similar to that reported for other Rhododendron species in which the procedure consists of in vitro multiplication of axillary shoots followed by rooting of the shoots utilizing ex vitro procedures (3, 5, 10). Although the basic procedures for many rhododendrons may be similar, differences have been reported in the manner by which particular species respond in culture. For example, optimum axillary shoot multiplication for Chapman's rhododendron ( Rhododendron chapmanii A. Gray) was achieved at a 2iP concentration of 2 ppm, (3) while for Flame azalea, 12 ppm was necessary (Fig. 1). Also, experience by the authors on micro-propagation of Chapman's rhododendron and Flame azalea have shown that, all factors being equal, the former will grow faster in culture and produce longer shoots than the latter. Similar observations have been reported by others and appear to be related to the great genetic diversity which exists within the genus (10). In the case of Chapman's rhododendron vs. Flame azalea, diversity is illustrated by the former being lepidote (scaly), evergreen and having a semi-indeterminate growth habit while the latter is elepidote (non-scaly), deciduous and exhibits determinate growth.
Significance to the Nursery Industry
Although the horticultural merits and beauty of Flame azalea have long been recognized, widespread use of this species has been limited due to difficulties regarding vegetative propagation. However, selection and vegetative propagation by micropropagation of superior selections is now feasible utilizing procedures described herein.
Literature Cited
1. Anderson, W.C. 1975. Propagation of rhododendron by tissue culture: Part I. Development of a culture medium for multiplication of shoots.
Proc. Intern. Plant Prop. Soc.
25:129-135.
2. Anderson, W.C. 1978. Rooting of tissue cultured rhododendrons.
Proc. Intern. Plant Prop. Soc.
28:135-139.
3. Blazich, F.A., C.C. Giles and C.M. Haemmerle. 1986. Micropropagation of
Rhododendron chapmanii. J. Environ. Hort.
4:26-29.
4. Economou, A.S. and P.E. Read. 1986. Micro-cutting production from sequential reculturing of hardy deciduous azalea shoot tips.
HortScience
21:137-139.
5. Fordham, I., D.P. Stimart and R.H. Zimmerman. 1982. Axillary and adventitious shoot proliferation of Exbury azaleas
in vitro. HortScience
17:738-739.
6. Kyte, L. and B. Briggs. 1979. A simplified entry into tissue culture production of rhododendrons.
Proc. Intern. Plant Prop. Soc.
29:90-95.
7. Li, H-L. 1957. Chromosome studies in the azaleas of eastern North America.
Amer. J. Bot.
44:8-14.
8. Liberty Hyde Bailey Hortorium. 1976.
Hortus Third: A Concise Dictionary of Plants Cultivated in the United States and Canada.
3rd ed. Macmillan Publishing Co., New York.
9. Lloyd, C. and B. McCown. 1980. Commercially-feasible micropropagation of mountain laurel,
Kalmia latifolia
, by use of shoot-tip culture.
Proc. Intern. Plant Prop. Soc.
30:421-437.
10. McCown, B.H. and G.B. Lloyd. 1983. A survey of the response of
Rhododendron
to
in vitro
culture.
Plant Cell, Tissue and Organ Culture
2:77-85.
11. Meyer, M.M. Jr. 1982.
In vitro
propagation of
Rhododendron catawbiense
from flower buds.
HortScience
17:891-892.
12. Nolde, S. and J. Coartney. 1985. Clonal variation in rooting of
Rhododendron calendulaceum
.
HortScience
20:539. (Abstr.)
13. Shelton, I.E. and R.E. Bir. 1980. Propagation of Flame azalea by softwood cuttings.
Proc. Southern Nurserymen's Assoc. Res. Conf., 25th Annu. Rept.
p. 220-221.
14. Skinner, H.T. 1961. Comments on the propagation of native azaleas.
Proc. Intern. Plant Prop. Soc.
11:96-98.
Frank A. Blazich is a Professor in the Department of Horticultural Science, North Carolina State University, Raleigh, North Carolina. His article "Micropropagation of Rhododendron chapmanii appeared in the ARS Journal Vol. 41 no.1.

