JARS v62n3 - Physiology of Cold-hardening in Rhododendron: Role of a Dehydrin Protein from R. catawbiense in Cryoprotection and Improving Freezing Tolerance
Physiology of Cold-hardening in
Rhododendron: Role of a Dehydrin Protein from R. catawbiense in Cryoprotection and Improving
Freezing Tolerance
Rajeev Arora and Yanhui Peng
Department of Horticulture,
Iowa State University
Dale Karlson
Division of Plant and Soil Sciences, West
Virginia University
José L. Reyes and Alejandra A. Covarrubias
Instituto de Biotecnología, UNAM, Cuernavaca, Mor., Mexico
Synopsis
Cold hardening is a physiologically complex trait, involving myriad of structural, physiological and biochemical adjustments by plant tissues (cell wall structure, membrane stability, enzymes activities, dehydration tolerance, carbohydrate status, to name few) that together play a role in improving freezing tolerance. Accumulation of hydrophilic proteins (dehydrins) that presumably act as tiny "sponges" has been widely observed in plant tissues during seasonal cold acclimation. It is argued that dehyrins help retain the hydration around cell membranes, a critical requirement for their structural integrity, and thereby protect plant cells from freeze-desiccation injury. Here we have investigated the role of a rhododendron dehydrin (RcDhn5) in conferring freezing tolerance. Although it is not clear what proportion of the "overall" freezing tolerance in R. catawbiense leaves might be contributed by RcDhn5, our results suggest that it may be one of the important pieces of the puzzle. We show that purified RcDhn5 rescues the activity of a cold sensitive enzyme LDH (lactate dehydrogenase) during a freeze-thaw stress. We also demonstrate that transgenic Arabidospis plants carrying high levels of active RcDhn5 gene are more freeze-tolerant than non-transgenic controls.
Introduction
For the past several years, our lab has been conducting research to understand the physiology and genetics of freezing tolerance and cold hardening in Rhododendron funded, in part, by the ARS Research Foundation. This research involved populations derived from a "very hardy" x "moderately hardy" parental cross ( Rhododendron catawbiense x R. fortunei ) in which progeny segregated for cold hardiness, measured as leaf-freezing tolerance (Lim et al., 1998 a, b). Based on our detailed investigation of the leaf proteins isolated from the non-hardened (August/summer) and cold–hardened (January/winter) plants of these parents, segregating progenies, and a wide array of Rhododendron species and cultivars, we demonstrated that 1) members of a specific class of proteins, called "dehydrins" generally accumulate at higher levels in hardened leaf tissues, and 2) accumulation of a particular dehydrin (of approximately 25 kDa molecular mass) was tightly linked to the degree of leaf freeze-tolerance and the cold-hardening ability (Lim et al., 1999; Marian et al., 2003). We hypothesized that this protein presumably played a key role in conferring freezing tolerance in Rhododendron .
Dehydrins are hydrophilic (water-loving) proteins, i.e., they serve the role of tiny "sponges" inside the plant cells that help retain the hydration around cell membranes and proteins thereby protecting them from desiccation injury. Numerous studies show that dehydrins accumulate at high levels during dehydrative stress and also during controlled (in growth-chambers) and/or natural (seasonal) cold hardening in most plant species, including woody plants (Arora et al., 1997; Dhanaraj et al., 2005; Kalberer et al. 2007; Karlson et al., 2003; Rinne et al., 1999; Wisniewski et al., 2006). It is argued that since freezing stress in plants results in dehydration due to the removal of cellular water to ice crystals out side the cell, protection of cells from such desiccation should be one of the key components of the cold hardening mechanisms during fall and winter. And, an accumulation of dehydrins is perhaps one way to achieve this protection. It is presumed that dehydrins serve as "cryoprotectants" whereby they protect cell membranes and other proteins from freeze-desiccation-induced perturbations in structure and function (Svensson et al., 2002).
Here we report our results from biochemical assays to test the cryoprotective ability of R. catawbiense dehydrin (RcDhn5, see below) and from experiments whereby the gene encoding RcDhn5 was inserted and expressed in a model plant Arabidopsis followed by testing and comparing the freezing tolerance of transgenic and non-transformed (control) Arabidopsis plants. Our results from biochemical assays indicate that purified RcDhn5 protects certain proteins against freeze-thaw stress-deactivation, and that transgenic Arabidopsis plants are more freeze-tolerant than their non-transformed counterparts.

|
Materials and Methods
IDENTIFICATION OF RcDhn5 gene.
One of the goals of our research program is to understand the physiological/genetic mechanism of freezing tolerance in Rhododendron . To that end, we initiated a "genomics" study to identify and isolate cold-hardening associated genes, including dehydrins, from rhododendron leaf tissues and to study their functions visà-vis freezing tolerance (Wei et al., 2005). Genomics involves a relatively large-scale study of gene sequences in living organisms (in contrast with one gene at a time) and being able to read and interpret them. Basically, we first constructed two cDNA (complementary DNA) libraries—assemblage of all the active (expressed) genes at a given time-point—from non-hardened (summer-sampled) and cold-hardened (winter-sampled) leaf tissues of a super-hardy species, Rhododendron catawbiense ; leaf freezing tolerances of the two samples are ~-6 °C and ~-50 °C, respectively (Lim et al., 1999; Wei et al., 2005). We then randomly selected ~500 cDNAs from each of the two libraries and determined their sequences - order of the nucleotide bases, adenine, guanine, cytosine, and thymine that are the building blocks of DNA molecule; these cDNAs may either be segments of genes or full-length genes. The sequence of a gene encodes and determines which protein a particular gene will produce and thus forms the basis for plant growth and development. Assemblage of all the sequence information from a set of cDNAs is called EST (expressed sequence tag) data set.
Using various bioinformatic analysis tools, we assembled these ESTs into various functional categories, such as photosynthesis-related genes, stress-responsive genes, water-transport-related genes, etc., based on their similarities with other known genes found in the databases available on the public domains (Wei et al., 2005). The relative frequencies at which specific cDNAs (genes) were randomly "picked" as ESTs from the respective cDNA libraries helped us to identify those genes that were preferentially expressed in non-hardened or cold-hardened tissues. For example, if 5 ESTs for a gene were detected in a set of 500 ESTs from cold acclimated tissues compared to only one EST for that gene from 500 ESTs of non-hardened tissues, this indicates that the gene was preferentially expressed in cold hardened leaves and could be functionally associated with cold hardening.
Our EST analysis revealed 5 distinct full-length dehydrin genes in the cold hardened cDNA library while only one from the non-hardened one; dehydrins belong to a multi-gene family, i.e., many dehydrin genes encode distinct dehdyrin proteins varying in size and sequence. We have since obtained full nucleotide sequences for all 5 dehydrin genes (RcDhn 1-5; (for R. catawbiense Dehydrin # 1-5). Using bioinformatic software, we have also determined amino acid sequence, molecular mass, and the isoelctric points (measure of acidic or basic nature) of these five dehydrins. Our results indicate that only RcDhn5 had the molecular mass close to 25 kDa (deduced mass = 29 kDa), while other 4 dehydrins were smaller or larger than 25 kDa by 13-22 kDa (unpublished results). These observations led us to speculate that perhaps RcDhn5 gene encodes for the 25 kDa dehydrin that had been the focus of previous cold hardiness studies by our lab (Lim et al., 1999; Marian et al., 2003). RNA blot experiments indicated that RcDhn5 gene was indeed highly abundant in cold hardened (winter-collected) leaf tissues of R. catawbiense , whereas it was present at substantially low levels in unhardened (summer-collected) and de-hardened (spring-collected) tissues (data not shown); RNA blot, also called Northern blot, is a laboratory technique routinely used to detect an active gene and its abundance in a biological sample at a given time. Therefore, experiments were conducted to isolate the dehydrin protein encoded by RcDhn5 (to be used for cryoprotection assays) and to create transgenic Arabidopsis plants containing RcDhn5 gene (to be used for freezing tolerance assays). Arabidopsis (instead of rhododendron) was chosen for transgenic experiments due to the unavailability of a successfully working procedure to create transgenic rhododendron plants (Knapp et al., 2001).
EXPRESSION OF RcDhn5 GENE IN E. coli BACTERIUM AND ISOLATION / PURIFICATION of RcDhn5 PROTEIN.
First step in this process is to insert the desired gene (in this case RcDhn5) into an appropriate vector, such as pGEX 6p3; a vector is a circular piece of DNA which has been genetically engineered to allow the insertion of desired foreign gene(s) in it with the help of restriction enzymes that chop off DNA at highly specific sites, a requirement to engineer a gene for successful insertion in plant tissues. pGEX 6p3 vector specifically carries a gene called GST which makes a protein Glutathione-S-transferase; RcDhn5 gene was inserted into this vector just ahead of the GST gene. The resultant "construct" (vector + the two genes fused together) was transferred into a specific strain of E. coli . Transformation of E. coli was confirmed by PCR (polymerase chain reaction)-based molecular tests; PCR technology is widely used to detect genes or gene fragments. Transformed E. coli was then cultured in a specific medium and induced by IPTG (a sugar that induces GST production) to make a fusion protein, i.e., GST + RcDhn5. This was confirmed by extracting bacterial proteins and separating them on an electrophoresis gel, a method whereby individual proteins (in a mixture) can be separated by their mass and visualized as bands on a gel matrix. The GST-RcDhn5 fusion protein was then separated from the total protein extract (also containing other bacterial proteins) by a technique called affinity chromatography. Finally, GST protein was removed from the RhDhn5 by a specific enzyme allowing the isolation of ample quantities of pure RhDhn5 protein.
CRYO-PROTECTION ASSAYS FOR RcDhn5.
Whether RcDhn5 dehydrin can protect other proteins/enzymes against freeze-thaw deactivation was determined by a biochemical assay, known as cryoprotection test (Lin et al., 1992; Reyes et al., 2005). Conceptually, this test involves subjecting a particular enzyme that is known to be cold/freeze-sensitive (such as LDH; lactate dehydrogenase) to a freeze-thaw treatment either in the presence of a test protein, such as RcDhn5, or without it (i.e., in the presence of a buffer minus the test protein) and measuring the residual activity of LDH following freeze-thaw cycle(s) (Lin et al., 1992). RcDhn5 would be deemed to have cryoprotective property if the loss in LDH activity after freeze-thaw is less in the presence of RcDhn5 than without it, when compared to no-freeze-thaw controls.
OVER-EXPRESSION OF RcDhn5 IN Arabidopsis thaliana . Arabidopsis is widely used in plant biology research including testing of gene functions via transformation experiments, i.e., creating transgenic plants carrying a gene of interest. Arabidopsis ' relatively short life cycle (seed to seed in ~ 10 weeks) makes it a highly desirable and efficient "model" plant for transformation studies where the seeds of transgenic plants can be obtained in a short time. We used "flower-dipping" method (Clough and Bent, 1998), as briefly described below, to transform Arabidopsis by RcDhn5 gene: 1) Non-dormant seeds (4-d "moist-chilled" in water at 4 °C) of Arabidopsis thaliana (ecotype: Columbia) were planted in peat-lite mix. Plants were grown in the greenhouse and allowed to flower; 2) Agrobacterium tumefaciens bacterial strain carrying RcDhn5 gene under a strong promoter, a DNA sequence that regulates the expression of gene, was prepared and its veracity was tested by antibiotic-resistance assay; Agrobacterium , due to its unique genetic properties, is typically used as a vehicle to carry and transfer a foreign gene of interest, such as RcDhn5, into a target plant tissue; 3) above-ground parts (particularly flowers) of Arabidopsis plants were dipped in the Agrobacterium solution (including Silwet L-77, a wetting agent, at 0.05%) for 2-3 sec with gentle agitation; 4) dipped plants were placed under a plastic dome for ~24 h to maintain high humidity; thereafter, plants were grown normally; 5) watering was withheld when seeds matured and dry seeds were harvested and planted on a selection medium to test whether germinating seedlings indeed carried RcDhn5; 6) transgenic plants, i.e., those carrying RcDhn5, were identified and selected using antibiotic-resistance of these seedlings and also by polymerase chain reaction (PCR); 7) transgenic plants were planted in the potting medium and maintained as before; 8) steps 5-7 were repeated to produce T2 plants (second generation of transgenic plants); 9) 3-4 week-old T2 plants were used to evaluate freezing tolerance and compared with non-transformed Arabidopsis plants.
FREEZING TOLERANCE OF TRANSGENIC (containing RcDhn5) AND NON-TRANSFORMED) Arabidopsis PLANTS.
Freezing tolerance of ~3 week old Arabidopsis plants was determined by adapting a laboratory-based test developed in our laboratory to evaluate leaf freezing tolerance in Rhododendron (Lim et al., 1998 a, b). Briefly, several replicates of Arabidopsis plants were cooled in a temperature-controlled glycol bath to a series of sub-freezing temperatures (after ice-nucleation) while unfrozen controls remained on ice. After over-night thaw, plants were gently shaken in de-ionized water and ion-leakage (indicator of freeze-thaw-induced membrane injury) was measured by a conductivity meter. Ion-leakage over a range of freezing temperatures was converted to "injury" estimates using the method used by our lab previously (Lim et al. 1998 a, b). Freeze-thaw injury responses of transgenic and non-transformed Arabidopsis plants were compared to determine if RcDhn5 conferred freezing tolerance.
Results and Discussion
RcDhn5 PROTECTS LDH ENZYME FROM FREEZE-THAW DEACTIVATION.
It has been previously reported that freeze-thaw reduces LDH activity and
an assay has been used in the past to measure the protective effect of
certain hydrophilic proteins on LDH activity during such a stress (Lin and
Thomashow, 1992). In our study, samples containing LDH, with or without
RcDhn5, were incubated in dry ice for 15 min and then thawed in a water
bath at 25°C for another 15 min (Fig. 1). To increase the chances of detecting
possible adverse effects, this two-step cycle was repeated up to 3 times before
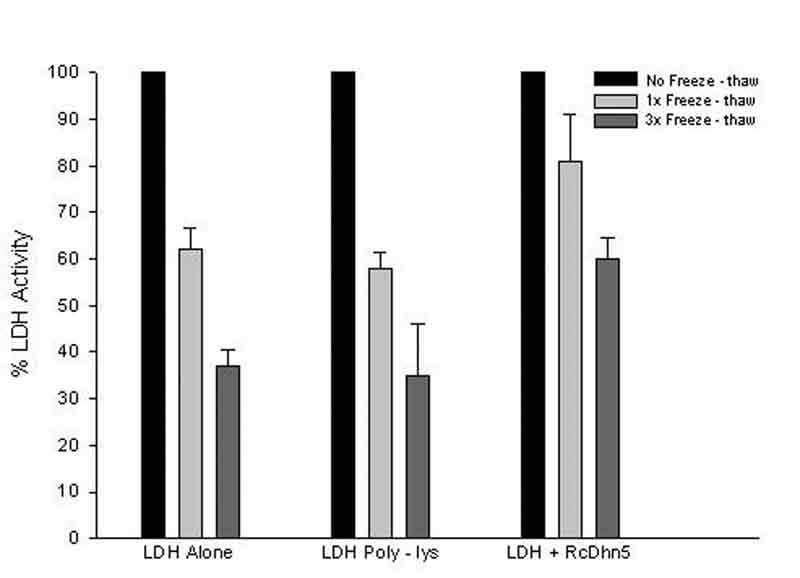
determination of residual LDH activity. Figure 2 shows the loss of LDH activity
when this enzyme was exposed to one or three cycles of freeze-thaw in the presence
or absence of RcDhn5. Data indicated that LDH in the absence of RcDhn5 lost
~38% or ~ 63% activity after one or three freeze-thaw cycles, respectively.
However, in the presence of RcDhn5, LDH activity was ~ 81% and 60%, respectively,
after identical treatments, i.e., ~30 – 62% higher residual LDH activity remained
in the presence of RcDhn5 (Fig. 2). It is noteworthy that parallel LDH
cryoprotection assays were conducted where a highly hydrophilic polypeptide,
poly L-Lysine, was used as an additive to LDH. Data indicated that poly L-Lysine
did not protect LDH above the LDH-alone (background) levels (Fig. 2). This
indicated that RcDhn5 protected LDH from freeze-thaw deactivation and that
this cryoprotection was a specific response. Our finding is consistent with
reports on cryoprotection of LDH by dehydrins from other plant species such as,
peach,
Arabidopsis
, barley, and winter wheat (Bravo et al., 2003; Houde
et al., 1995; Lin and Thomashow 1992; Wisniewski et al., 1999).
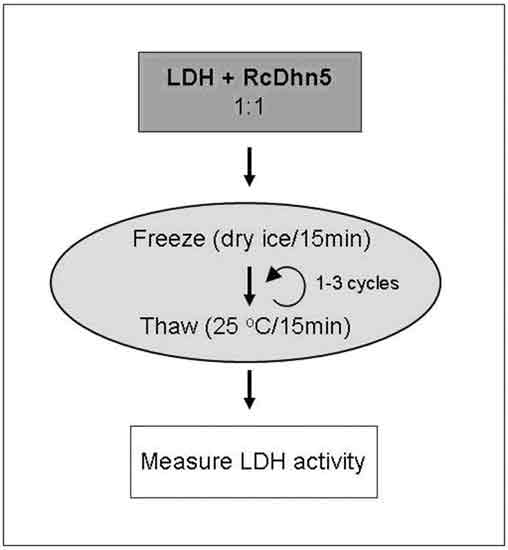
|
|
Figure 1. Flow chart of the experimental design
for an assay to monitor the
effects of freeze-thaw on LDH (lactate dehydrogenase) enzyme activity in the presence or absence of RcDhn5. Samples containing LDH without or with RcDhn5 protein (in 1:1 ratio; 250 nM final concentration in a Tris buffer at pH 7.5) were incubated in dry ice for 15 min and then moved to a water bath at 25ºC for 15 min. One or three identical cycles were performed and samples were returned to ice. LDH activity was determined before freeze-thaw (unstressed LDH) and immediately after freeze-thaw treatment (for the remaining activity following stress). |
Mechanism by which dehydrins cryoprotect LDH is not well known. However, it has been suggested (Close, 1997) that specific stretches of aminoacids (molecules that join together to make a protein) within dehydrin protein might be key to dehydrins' cryoprotective ability. These "stretches" (domains) are known to be conserved in all plant dehydrins and possess unique structural properties such as containing both polar and non-polar binding surfaces. It is argued that these domains interact with exposed patches of LDH or with membrane lipids in the case of plant cells that might have been structurally perturbed (partially unfolded) after the freeze-thaw, and prevent further unfolding and inactivation (Koag et al., 2003).
|
|
Figure 2. Samples containing LDH (lactate dehydrogenase) with or without RcDhn5 and
Poly-L-Lysine were frozen and
thawed once (1x) or three (3x) times (as in Figure 1) and LDH activity determined immediately after thawing. Percent values are with respect to the LDH activity without freeze-thaw treatment taken as 100 percent. |
As mentioned earlier, dehdyrins typically accumulate to high levels in plant tissues during cold hardening. Some evidence indicates this accumulation to be around plasma membrane of cells (Danyluk et al., 1998; Puhakainen et al., 2004). Garay-Aroyo et al. (2000) suggested that dehydrins, due to their highly hydrophilic nature, essentially act as tiny "sponges" that help retain the hydration around cell membranes (this hydration is critical for their structural integrity) and thereby protect plant cells from freeze-desiccation injury. Rinne et al. (1999) showed that a birch ( Betula pubescens ) dehydrin was able to improve the activity of alpha-amylase enzyme under conditions of low water activity. They hypothesized that the birch dehydrin might create local pools of water in cold-hardened stem tissues and allow alpha-amylase to remain active (by providing critically needed hydration) and convert starch to sugars, a physiological change typically observed in tissues during seasonal cold hardening. However, our results from the comparative cryoprotection assays for RcDhn5 and poly-L-Lysine (Fig. 2) suggest that hydophilicity alone might not be sufficient for RCDhn5's protective ability (though it might still be necessary), and that features other than or in addition to hydrophilicity might be key to RcDhn5's cryoprotective role.
|
|
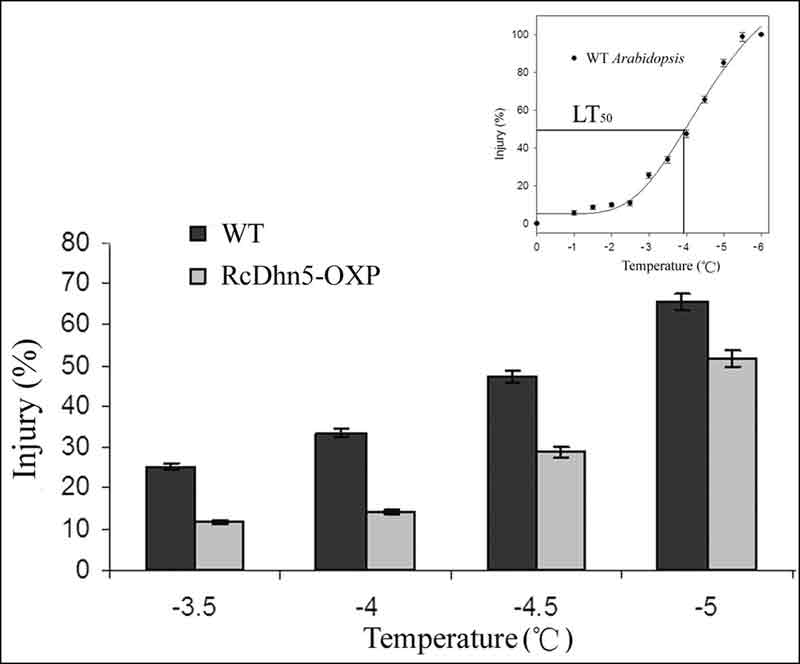
Figure 3. Freezing tolerance of RcDhn5-OXP (genetically engineered to over-express
RcDhn5 gene) and
non-transformed (wild-type – WT) Arabidopsis plants in response to a laboratory freeze-thaw treatment. Inset: A typical freeze-thaw injury response curve for Arabidopsis ; LT50 denotes the temperature causing 50% injury. |
RcDhn5 IMPROVES FREEZING TOLERANCE OF TRANSGENIC Arabidopsis PLANTS.
Freeze-thaw injury in plant tissues (estimated from ion-leakage) over a range of treatment temperatures typically displays a sigmoidal (shaped like the letter "S") response (Lim et al., 1998 a., b). Similar response curve for Arabidopsis indicates that unhardened plants are minimally injured up to -3 °C and maximally injured at ~-6 °C with incremental injury occurring between -3 C and -5 C (Fig 3 ‘inset'). Therefore, we chose -3.5, -4, -4.5, and -5 °C as test temperatures for comparing the freezing tolerance (FT) response of non-transformed Arabidopsis plants with that of transgenic ones carrying RcDhn5 gene. Our data indicated that the relative level of freeze-thaw injury of Arabidopsis plants transformed with RcDhn5 (when exposed to -3.5, -4, -4.5, and -5 ºC) was significantly lower (by ~50%, 58%, 38%, and 18% respectively) than that of the non-transformed controls (Fig. 3), indicating that RcDhn5 indeed contributed to FT in Arabidopsis plants.
Our results, in general, are similar to that of Puhakainen et al. (2004) who too observed enhanced FT of Arabidopsis plants over-expressed with dehydrin genes except for two notable differences: one, that these authors made transgenic plants containing two dehydrin genes instead of one as in our study, and two, that the dehydrins genes, which were made to express at much higher than normal levels in transgenic Arabidopsis , were indeed Arabidopsis dehydrins as opposed to Rhododendron dehydrin as in our study. Using sophisticated microscopy techniques and antibodies against these specific dehydrins, these authors also noted that the over-reproduced dehydrins (due to the over-expression of the genes) accumulated in the vicinity of membranes of Arabidopsis leaf cells. Another study by Hara et al. (2003) noted enhanced cold tolerance of transgenic tobacco plants over-expressing a Citrus unshiu dehydrin gene. How exactly these dehydrins confer frost tolerance to plant tissues is not clear. However, above discussion related to cryoprotection property of dehydrins coupled with their putative role in membrane stabilization (due to the accumulation of certain dehydrins close to cell membranes) point to their role in preventing structural damage to membranes and maintaining activity of key enzymes during freeze-induced cellular dehydration.
Concluding Remarks
From the perspective of basic research, identification of the genes responsible for conferring freezing tolerance would be highly useful in breeding programs for "marker-assisted" selection of cold-hardy genotypes where the presence or absence of certain gene/DNA fragment, visualized as bands on electrophoresis gels, can "mark" or distinguish between cold hardy and tender genotype even at a young age. Moreover, such knowledge could be used to develop new selections by genetically engineering improved cold hardiness into genotypes with ornamentally superior traits. Finally, investigating the physiological factors responsible for freezing/desiccation tolerance will also advance our knowledge of the biology of plant's response to these stresses. Future experiments to determine dehydration tolerance of RcDhn5-expressing transgenic Arabidopsis plants and assay RcDhn5's ability, if any, to protect LDH against dehydration stress would help us to further elucidate the functional importance of this dehydrin in freeze-desiccation tolerance.
Acknowledgements
This research was supported, in part, by a grant from the Research Foundation of American Rhododendron Society and Iowa State University Agriculture and Home Economics Experiment Station.
The Authors
Dr. Rajeev Arora (corresponding author) is a Professor and Dr. Yanhui Peng is postdoctoral researcher in the Department of Horticulture at Iowa State University, 139 Horticulture Hall, Ames, IA 50011. Dr. Dale Karlson is an Assistant Professor in the Division of Plant and Soil Sciences at West Virginia University, P.O. Box 6108, Morgantown, WV 26506. Dr. José L. Reyes is a postdoctoral researcher and Dr. Alejandra A. Covarrubias is a Professor of Biochemistry in the Instituto de Biotecnología, UNAM, Cuernavaca, Mor., Mexico.
Literature Cited
Arora, R., L. J. Rowland and G. R. Panta. 1997. Chill-responsive dehydrins in blueberry: Are they associated with cold hardiness or dormancy transitions? Physiologia Plantarum , 101:8-16.
Bravo, L. A., J. Gllardo, A. Navarrete, N. Olave, J. Martinez, M. Alberdi, T. J. Close and L. J. Corcuera. 2003. Cryopritective activity of a cold-induced dehydrin purified from barley. Physiologia Plantarum , 118:262-269.
Close, T. J. 1997. Dehydrins: a commonalty in the response of plants to dehydration and low temperature. Physiol. Plant ., 100:291-296.
Clough, S.J. and A.F. Bent.1998. Floral dip: A simplified method for Agrobacterium -mediated transformation of Arabidopsis thaliana . Plant J. , 16:735-743.
Danyluk, J., A. Perron, M. Houde, A. Limin, B. Foweler, N. Benhamou, and F. Sarhan. 1998. Accumulation of an acidic dehydrin in the vicinity of the plasma membrane during cold acclimation of wheat. Plant Cell , 10:623-638.
Dhanaraj, A. L., J. P. Slovan and L. J. Rowland. 2005. Isolation of a cDNA clone and characterization of expression of a highly abundant, cold acclimation-associated 14 kDa dehdyrin in blueberry. Plant Sci. , 168:949-957.
Garay-Arroyo, A., J. M. Colmenero-Flores, A. Garciarrubio and A. A. Covarrubias. 2000. Highly hydrophilic proteins in prokaryotes and eukaryotes are common during conditions of water-deficit. J. Biol. Chem. , 275:5668-5674.
Hara, M., S. Terashima, T. Fukaya and T. Kuboi. 2003. Enhancement of cold-tolerance and inhibition of lipid peroxidation by citrus dehydrin in transgenic tobacco. Planta , 217:290-298.
Houde, M., C. Daniel, M. Lachapelle, F. Allard, S. Laliberte and F. Sarhan.1995. Immunolocalization of freezing-tolerance-associated proteins in the cytoplasm and nucleoplasm of wheat crown tissues. Plant J. , 8:583-593.
Kalberer, S. R, N. Leyva-Estrada, S. L. Krebs and R. Arora. 2007. Frost dehardening and rehardening of floral buds of deciduous azaleas depend on genotypic biogeography. Environmental & Experimental Botany , 59:264-275.
Karlson, D. T., Y. Zheng, V. E. Stirm, R. J. Joly and E. N. Ashworth. 2003. Phoperiod regulation of a 24-kDa dehydrin-like protein in red-osier dogwood ( Cornus sericea L.) in relation to freeze-tolerance. Plant Cell Physiol. , 44:25-34.
Knapp, J. E., A. P. Kausch, C. Auer and M. H. Brand. 2001. Transformation of Rhododendron through microprojectile bombardment. Plant Cell Rep. 20:749-754.
Koag, M-C., R. D. Fenton, S. Wilkens and T. J. Close. 2003. The binding of Maize DHN1 to lipid vesicles. Gain of structure and lipid specificity. Plant Physiol. 131:309-316.
Lim, C-C., R. Arora and S. L. Krebs. 1998 a. Genetic study of freezing-tolerance in Rhododendron populations: Implications for cold hardiness breeding. J. Amer. Rhodo. Soc. (summer):143-148.
Lim, C-C., R. Arora and E. C. Townsend. 1998 b. Comparing Gompertz and Richards functions to estimate freezing injury in Rhododendron using electrolyte leakage J. Amer. Soc. Hort. Sci. , 123 (2):246-252.
Lim, C-C., S.L. Krebs, and R. Arora. 1999. A 25 kD dehydrin in association with age- and genotype-dependent leaf freezing tolerance in Rhododendron . Theo. Appl. Gen. , 99:912-920.
Lin, C. and M.F. Thomashow. 1992. A cold-regulated Arabidopsis gene encodes a polypeptide having potent cryoprotective acitivity. Biochem. Biophys. Res. Commun. ,183:1103-1108
Marian, C.O., S.L. Krebs and R. Arora. 2003. Dehydrin variability among Rhododendron spp: A 25 kDa dehydrin is highly conserved and associated with cold acclimation across wide array of species. New Phytol. , 161 (3):773-780.
Puhakainen, T., M. W. Hess, P. Makela, J. Svensson, P. Heino and E. T. Palva. 2004. Overexpression of multiple dehydrin genes enhances tolerance to freezing stress in Arabidopsis . Plant Mol Biol. , 54:743-753.
Reyes, J.L., M-J. Rodrigo, J.M. Colmenero-Flores, J-V. Gil, A. Garay- Arroyo, F. Campos, F. Salamini, D. Bartels, and A.A. Covarrubias. 2005. Hydrophilins from distant organisms can protect enzymatic activities from water limitation effects in vitro, Plant Cell and Environ. , 28:709-718.
Rinne, P. L. H., P. L. M. Kaikuranta, L. H. W. Van der Plas and C. Van der Schoot. 1999. Dehydrins in cold-acclimated apices of birch ( Betula pubescens Ehrh.): production, localization and potential role in rescuing enzyme function during dehydration. Planta , 209:377-388.
Svensson, J., A.M. Ismail, T. Palva and T.J. Close. 2002. Dehydrins: In Storey, K.B. and Storey, J.M. (eds.), Sensing, signaling, and cell adaptation, Elsevier Science BV, 155-171.
Wei, H., A.L. Dhanaraj, L.J. Rowland, Y. Fu, S.L. Krebs and R. Arora. 2005. Comparative analysis of expressed sequence tags (ESTs) from coldacclimated and non-acclimated leaves of Rhododendron catawbiense Michx. Planta , 221:406-416.
Wisniewski, M. E., T. J. Close, T. Artlip and R. Arora. 1996. Seasonal patterns of dehydrins and 70-kDa heat-shock proteins in bark tissues of eight species of woody plants. Physiol. Plant. , 96:496-505.
Wisniewsk, M., R. Webb, R. Balsamo, T. J. Close, X. M. Yu and M. Griffith. 1999. Purification, imunolocalization, cryoprotective, and antifreeze activity of PCA60: a dehydrin from peach ( Prunus persica ). Physiol. Plant. , 105:600-608.
