JARS v62n3 - A Novel Method for Inducing Poyploidy in Rhododendron Seedlings
A Novel Method for Inducing Poyploidy
in Rhododendron Seedlings
Jeff R. Jones, Thomas G. Ranney, and Thomas A. Eaker
Department of Horticultural Science, Mountain Horticultural Crops Research and Extension Center
North Carolina State University, Fletcher, North Carolina
Introduction
Polyploidy (having three or more complete sets of chromosomes) is relatively common in plants. By some estimates as many as 70% of all angiosperms are natural polyploids (Masterson, 1994). The importance and prevalence of polyploidy is especially evident in the genus Rhododendron . Polyploidy occurs naturally in many species and hybrids of Rhododendron , particularly in the Rhododendron and Pentanthera subgenera (Ammal et al., 1950; Jones et al., 2007).
Polyploidy is considered to be a major pathway for plant evolution and can contribute to reproductive isolation and abrupt speciation (Ramsey and Schemske, 1998; Soltis et al., 2003; Wendel, 2000). The effects of polyploidy on plant traits are also important to horticulturists and plant breeders. Ploidy levels can influence crossability, fertility of hybrids, plant vigor, and gene expression (Ranney, 2006). The induction of artificial polyploids has been utilized in the development of allopolyploids to restore fertility in sterile hybrids, enhance crossability and fertility of progeny, create seedless triploids, produce novel gene combinations, and increase the expression and diversity of secondary metabolites (Chen and Ni, 2006; Contreras et al., 2007; Olsen et al., 2006; Soltis and Soltis, 1993; Wendel, 2000). In some cases, polyploids may also have additional desirable ornamental characteristics including thicker leaves and larger flowers with thicker petals that persist longer (Barlup, 2002; Hosoda et al., 1953; Kehr, 1996a; Leach, 1961).
Polyploidy can arise naturally through multiple pathways including spontaneous chromosome doubling in somatic meristem cells and sexual fertilization with unreduced gametes (Jones et al., 2007; Ramsey and Schemske, 1998; Widrlechner et al., 1982). Polyploidy can also be induced through the use of various chemical doubling agents (mitotic inhibitors) (Contreras et al., 2007; Sanford, 1983; van Tuyl, 1992). Colchicine (N-(5,6,7,9-tetrahydro-1,2,3,10-tetra-methoxy-9-oxobenzo(a)heptalen-7-yl)acetamide) was first discovered as an effective mitotic inhibitor in 1937 and has been extensively utilized for inducing polyploidy in a wide range of species (Eigsti and Dustin, 1955; Hancock, 1997). The dinitroaniline herbicide oryzalin (3,5-dinitro-N4,N4-dipropylsufanilamide) has also been effectively utilized as a doubling agent and is considerably less toxic than colchicine (van Tuyl, 1992). Both agents have a similar mode of action: inhibiting microtubule polymerization and arresting mitosis at metaphase thus preventing the replicated chromosomes from separating into daughter cells. When mitosis resumes, a lineage of polyploid cells with double the normal chromosome number can be established. Studies comparing the effectiveness of colchicine versus oryzalin as induction agents have produced mixed results. Oryzalin is more effective at lower dosages than colchicine due to a higher specificity for tubulin binding sites in plant material (Eeckhaut et al., 2001; Eiselein, 1994; Morejohn et al., 1987; van Tuyl, 1992). Undesirable side effects of colchicine, including sterility, abnormal growth, and deformed tissue can be avoided when using oryzalin (Bouvier, 1994; van Tuyl, 1992). Eeckhaut et al. (2001) found that in-vitro treatment of seedlings of rhododendron with 0.05% and 0.25% colchicine had no effect on ploidy while treatment with 0.01% and 0.05% oryzalin yielded some polyploids and numerous cytochimeras. Väinölä (2000) compared the efficacy of colchicine (0.025% or 0.05%) and oryzalin (0.001% or 0.005%) for 24 or 48 hour durations on chromosome doubling in rhododendron seedlings. Plant survival was higher with colchicine, but oryzalin was more efficient in the induction of polyploidy (18% of the surviving plants at 0.005% with the 24 hr exposure). The use of mitotic inhibitors often produces polyploids that are cytochimeras (mixaploids) whereby different cells or histogenic layers vary in ploidy level (Pratt, 1983; Pryor and Frazier, 1968; Väinölä, 2000). The ploidy of the LII histogenic layer is crucial to breeding as this layer gives rise to reproductive tissue (Pratt, 1983; Ranney, 2006).
Polyploidy has been induced in many woody ornamental plant genera such as xChitalpa (Olsen et al., 2006), Citrus (Lee, 1988), Rosa (Semeniuk and Arisumi, 1968), Prunus (Dermen, 1953), and Pyrus (Kadota and Niimi, 2002). Attempts to induce polyploidy in rhododendrons, through an assortment of methods both ex-vitro and in-vitro, have been met with varying degrees of success (Contreras et al., 2007; Eeckhaut et al., 2001; Eiselein, 1994; Kehr, 1996b; Paden, 1990; Pryor and Frazier, 1968; Sakai et al., 2004; Väinölä, 2000). Mitotic inhibitors only affect actively dividing cells; therefore, prolonged contact with the apical meristem is crucial for inducing polyploidy, yet overexposure results in death (Kehr, 1996a). Pryor and Frazier (1968) successfully applied colchicine to actively growing shoots to obtain tetraploid azaleas. Kehr (1996b) developed a protocol for misting seedlings with colchicine after the cotyledons developed but before the first true leaves were evident; however, efficacy of the treatment was never determined. Contreras et al. (2007) developed an allotetraploid form of Rhododendron 'Fragrant Affinity' by submerging actively growing shoots tips in 150μM oryzalin for 24 hours. Eiselein (1994) compared single applications of 1% colchicine for 0, 24, 48, 72, and 96 hours with repeated applications of a 24-hour exposure, interrupted by 2-5 day recovery periods, totaling 96 hours of treatment. Percentage of tetraploids (determined on root tips - no data presented on shoots) with the single treatments averaged approximately 20% with no effect of duration, while the repeated applications resulted in a 79% conversion rate. The higher conversion rate for the repeated applications may have resulted from impacting a greater number of cells in metaphase, over time, while allowing for periodic recovery periods (Eiselein, 1994).
Confirmation of ploidy levels in treated seedlings is essential to determine efficacy of these techniques. Although determination of ploidy level in Rhododendron by counting chromosomes is possible, the chromosomes of Rhododendron are small and particularly difficult to view and discern using standard cytological techniques (Eiselein, 1994; Tolstead and Glencoe, 1991). Flow cytometry provides a fast and efficient method for determining relative genome size and associated ploidy level of Rhododendron (De Schepper et al., 2001; Eeckhaut et al., 2004; Jones et al., 2007). An additional advantage of flow cytometry for evaluating efficacy of mitotic inhibitors is the ability to sample thousands of cells and also determine the presence of cytochimeras (De Schepper et al., 2001; Jones et al., 2007).
The objectives of this project were to 1) develop a simple and effective, ex-vitro method for inducing polyploidy in Rhododendron seedlings, 2) evaluate the effectiveness of using repeated treatments of an oryzalin suspension in a warm agar solution applied directly to apical shoots of Rhododendron seedlings to induce polyploidy, and 3) develop a population of new polyploid rhododendrons and azaleas for use in future breeding projects.
Materials and Methods
Controlled pollinations were completed to produce new hybrids with desirable ornamental characteristics for use in this study. All parents were confirmed diploids (Jones et al, 2007). Seeds were obtained from the following crosses:
1) R. 'Summer Lyric' ( R. prunifolium x R. arborescens ) [pollinated with either R. 'Millennium' (R. 'Weston's Sparkler'* x R. 'Weston's Parade'*) or R. 'August Beauty'* ( R. prunifolium x R. arborescens )] with the goal of producing tetraploid deciduous azaleas (subgenus Pentanthera ) with fragrant flowers, a range of flower colors, and late-season flowering.
2) R. 'Cheyenne' (R. Jalisco Group x R. Loderi Group) x R. 'Capistrano' (R. 'Hindustan' x {{[ R. catawbiense x ( R. fortunei ssp. discolor x R. Fabia Group)] x (R. 'Russell Harmon' x R. 'Goldsworth Orange')} x R. 'Golden Gala'}) with the goal of producing a tetraploid elepidote rhododendron (subgenus Hymenanthes ) with fragrant, yellow flowers.
3) R. 'Kimberly' ( R. williamsianum x R. fortunei ssp. fortunei ) x R. 'Nestucca' ( R. fortunei ssp. fortunei x R. degronianum ssp. yakushimanum ) with the goal of producing a tetraploid elepidote rhododendron (subgenus Hymenanthes ) with a compact habit, good cold hardiness, and fragrant flowers.
Seedlings from each cross were germinated in five separate pots with approximately 100 seeds per pot. When seedlings were at the cotyledon stage, all of the plants (subsamples) in an individual pot were either treated with 1, 2, 3, or 4 applications of oryzalin separated by 4-day intervals or left untreated (control). The pre-emergent herbicide Surflan® A.S. (40.4% oryzalin) was diluted to produce a suspension containing 50μM oryzalin with 5.5g/L agar at 50 °C. Concentrations of oryzalin and agar were based on preliminary studies (data not presented). A single drop (2-4 μL) of warm (~40 °C) oryzalin suspension was then pipetted on top of the cotyledons of each seedling to cover the emerging shoot. Pots were placed in a high humidity (approximately 100% relative humidity) growth chamber at 23ºC under constant light to preserve the integrity of the agar droplet. Subsequent applications were made after the 4-day interval. After treatment, plants were grown under standard greenhouse conditions prior to analysis. The experimental design was completely randomized. Flow cytometry was utilized to determine ploidy levels approximately 3 months after treatment using methods described by Jones et al. (2007). Data on percent death and ploidy level were subjected to regression analysis (PROC REG; SAS version 8.02, SAS Institute., Cary, N.C.; SAS Institute, 1988).
Results and Discussion
The semi-solid agar appeared to be an effective medium for applying oryzalin to the apical shoots of rhododendron seedlings (Figure 1). The agar drop typically rested on the cotyledons and solidified around the elongating shoots, thus allowing for sufficient contact between the oryzalin and the meristem. Drops persisted for 2 to 3 days before deteriorating. There were no visual phytotoxic symptoms over the treatment periods.

|
| Figure 1. Oryzalin-agar treatment of hybrid Rhododendron seedling. |
Treatment of 'Summer Lyric' seed lings resulted in a broad range of ploidy levels including mixaploids (Table 1). Induction percentages for the different classes of polyploids varied as a function of number of applications. The percentage of homogeneous tetraploids (of primary interest) followed a quadratic trend in response to increasing number of applications with the highest percentage, 41%, resulting after two successive applications of oryzalin. A few higher level polyploids, including octoploids, and mixaploids were also recovered as the number of applications increased. Although a quadratic response was significant for seedling death, the increasing number of applications did not increase death over that of the control. Overall, it appeared that 2 to 3 applications were ideal for inducing tetraploids.
A few polyploids resulted from the oryzalin treated R. 'Cheyenne' x 'Capistrano' hybrids (Table 2). Mixaploids increased with number of applications, while there was no significant trend for tetraploids. The percentage of dead plants increased linearly with increasing application number, suggesting a sensitivity to oryzalin. Oryzalin treatments did result in some polyploids, but it remains unclear if there was any benefit from multiple applications to induce tetraploid plants.
| Table 1. Ploidy levels and death of seedlings from Rhododendron 'Summer Lyric' following treatment of apical shoots with 0, 1, 2, 3, or 4 applications of 50μM oryzalin in agar separated by 4 day intervals. | ||||||
| Number of Applications | ||||||
| Ploidy | 0 | 1 | 2 | 3 | 4 | Trend |
| 2x | 89 z | 59 | 24 | 31 | 19 | Q y ***; R2=0.95 |
| 2x+4x | 0 | 21 | 26 | 26 | 31 | Q**; R2=0.92 |
| 4x | 0 | 12 | 41 | 33 | 24 | Q***; R2=0.86 |
| 4x+8x | 0 | 0 | 4 | 0 | 8 | L X **; R2=0.49 |
| 8x | 0 | 0 | 1 | 2 | 0 | NS W |
| 2x+8x | 0 | 0 | 1 | 0 | 5 | L**; R2=0.53 |
| 2x+4x+8x | 0 | 0 | 0 | 0 | 3 | Q*; R2=0.85 |
| Dead | 11 | 8 | 2 | 9 | 10 | Q*; R2=0.66 |
|
Z
Data in percent.
X
L=linear trend.
Y
Q=quadratic trend.
W
NS=trend not significant.
* significant, P ≤ 0.10 ** significant, P ≤ 0.05 *** significant, P ≤ 0.01 |
||||||
| Table 2. Ploidy levels and death of Rhododendron 'Cheyenne' x R. 'Capistrano' seedlings following treatment of apical shoots with 0, 1, 2, 3, or 4 applications of 50μM oryzalin in agar separated by 4 day intervals. | ||||||
| Number of Applications | ||||||
| Ploidy | 0 | 1 | 2 | 3 | 4 | Trend |
| 2x | 69 z | 59 | 24 | 31 | 19 | Q y ***; R2=0.88 |
| 2x+4x | 0 | 8 | 7 | 6 | 2 | Q**; R2=0.88 |
| 4x | 0 | 2 | 7 | 8 | 4 | NS w |
| Dead | 31 | 68 | 65 | 68 | 80 | L x ***; R2=0.70 |
|
Z
Data in percent.
Y
Q=quadratic trend.
X
L=linear trend.
W
NS=trend not significant.
* significant, P ≤ 0.10 ** significant, P ≤ 0.05 *** significant, P ≤ 0.01 |
||||||
| Table 3. Ploidy levels and death of Rhododendron 'Kimberly' x R. 'Nestucca' seedlings following treatment of apical shoots with 0, 1, 2, 3, or 4 applications of 50μM oryzalin in agar separated by 4 day intervals. | ||||||
| Number of Applications | ||||||
| Ploidy | 0 | 1 | 2 | 3 | 4 | Trend |
| 2x | 67 z | 30 | 43 | 33 | 20 | L x ***; R2=0.67 |
| 2x+4x | 0 | 6 | 9 | 11 | 11 | L**; R2=0.85 |
| 4x | 0 | 4 | 12 | 11 | 12 | L***;R2=0.81 |
| 4x+8x | 0 | 0 | 0 | 1 | 1 | NS w |
| 8x | 0 | 0 | 0 | 4 | 0 | NS |
| 2x+8x | 0 | 0 | 0 | 0 | 1 | NS |
| Dead | 33 | 60 | 36 | 40 | 55 | NS |
|
Z
Data in percent.
X
L=linear trend.
W
NS=trend not significant.
* significant, P ≤ 0.10 ** significant, P ≤ 0.05 *** significant, P ≤ 0.01 |
||||||
Among the R. 'Kimberly' x 'Nestucca' seedlings, oryzalin treatment resulted in a range of polyploids including tetraploids, several octoploids, and three classes of mixaploids (Table 3). Diploids decreased linearly with each additional application, and conversely, the percentage of 2x+4x mixaploids and solid tetraploids increased linearly. The octoploid, higher level mixaploids, and death percentages were random in their distribution with no significant trend. For induction of tetraploids, 2-4 applications were optimal.
|
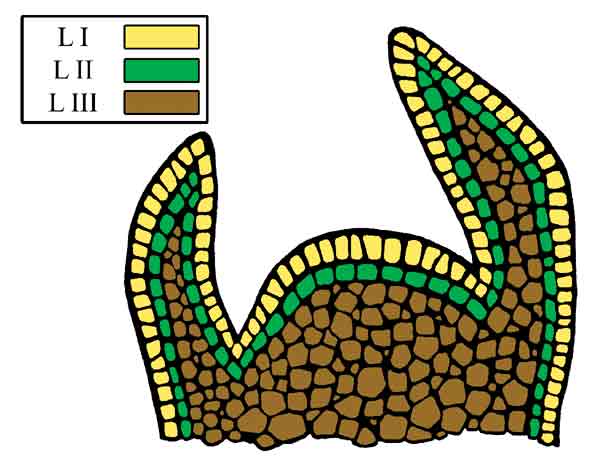
| Figure 2. Diagram of the shoot apical meristem highlighting the three histogenic layers. |
The shoot apical meristem (Figure 2) is comprised of zones. The central zone includes a group of cells at the distal end of the meristem. These cells function as intitial cells that give rise to other cells, other regions of the meristem, and ultimately the shoot (Francis, 1997; Kerstetter and Hake, 1997; Tax and Durbank, 2006). Within the central zone lay multiple histogenic layers: L1, L2, and L3, that are distinct and give rise to separate cell lines and tissues (Hudson and Goodrich, 1997). Development of homogeneous polyploids requires the successful doubling of the initial cells, in all histogenic layers, within the central zone of the apical meristem. In contrast, mixaploids appeared to be a conglomeration of cells of varying ploidy levels, among or within the histogenic layers, resulting from incomplete doubling of initial cells within the meristem. As suggested by Eiselein (1994), only a certain percentage of meristematic cells may be affected by any single application of a mitotic inhibitor. Pryor and Frazier (1968) also observed mixaploids following a single application of colchicine on evergreen azaleas. Poor penetration or asynchronous cell cycling within the meristem could result in only partial doubling of the meristem. A gradient of cell size, relative growth rates and cell cycling times can exist within a meristimatic zone (Francis, 1997). Because the cell cycle is not synchronized among all the cells in the meristem, multiple applications may induce polyploidy in different populations of cells. In some cases, e.g., 'Summer Lyric' and 'Kimberly' x 'Nestucca' seedlings, increasing the number of applications (from 2 to 3 or 1 to 2, respectively) increased the number of homogeneous tetraploids. Thus, repeated applications over time most likely allowed for doubling of different initial cells during several asynchronous cell cycles.
Stability of the mixaploids developed here and the specific nature of their chimeral arrangement is uncertain. If all the cells in an individual histogenic layer are uniformly one ploidy level, e.g., a periclinal chimera, the confirmation may be more stable. Limited sampling two months after initial testing revealed that many of the higher level mixaploids eventually reverted to their lower ploidy level (data not presented). Väinölä (2000) reported similar results in which one third of the induced mixaploids shifted to diploidy. If the meristem is composed of a mosaic of cells with different ploidy levels mixed within histogenic layers, some cell types may multiply faster (those cells of lower ploidy) and effectively overrun the other cell type (those of higher ploidy) in a phenomenon known as diplontic selection (Broertjes and Keen, 1980; Pratt, 1983). Cell types of higher DNA content typically take longer to cycle through mitosis (Singh, 1993) and selection will then favor reversion to the faster proliferating, lower ploidy level, cytotype. The higher level polyploids (e.g., octoploids) likely resulted from mitotic inhibition of multiple cell cycles whereby diploid meristematic cells became tetraploid, and those tetraploid cells were doubled again to become octoploid. Such occurrences have been previously noted in polyploid induction of apple (Tilney-Bassett, 1986).
The results of this study demonstrate that the method of applying a suspension of oryzalin in warm, semi-solid agar to the shoots of Rhododendron seedlings is an effective method for inducing polyploidy. Although single applications resulted in some polyploid plants, multiple applications increased efficacy for some of the taxa studied. Polyploid plants developed in this study will be further evaluated for desirable traits and incorporated into an ongoing rhododendron breeding program.
Literature Cited
Ammal, E.K.J., I.C. Enoch, and M. Bridgwater. 1950. Chromosome numbers in species of rhododendron. Rhododendron Year Book 5:78-91.
Barlup, J. 2002. Let's talk hybridizing: Hybridizing with elepidote polyploid rhododendrons. J. Am. Rhod. Soc. 76:75-77.
Bouvier, L., F.R. Fillon, and Y. Lespinasse. 1994. Oryzalin as an efficient agent for chromosome doubling of haploid apple shoots in vitro. Plant Breeding 113:343-346.
Broertjes, C. and A. Keen. 1980. Adventitious shoots: do they develop from one cell? Euphytica 29:73-87.
Chen, Z.J. and Z.F. Ni. 2006. Mechanisms of genomic rearrangements and gene expression changes in plant polyploids. BioEssays 28:240-252.
Contreras, R.N., T.G. Ranney, and S.P. Tallury. 2007. Reproductive behavior of diploid and allotetraploid Rhododendron L. 'Fragrant Affinity'. HortScience 42:31-34.
Dermen, H. 1953. Periclinal cytochimeras and origin of tissues in stem and leaf of peach. Amer. J. Bot. 40:154-168.
De Schepper, S., L. Leus, M. Mertens, E. Van Bockstaele, and M. De Loose. 2001. Flow cytometric analysis of ploidy in Rhododendron (subgenus Tsutsusi ). HortScience 36:125-127.
Eeckhaut, T., G. Samyn, and E. Van Bockstaele. 2001. In vitro polyploidy induction in Rhododendron simsii-hybrids. Med. Fac. Landbouww. 66:451-454.
Eeckhaut, T.G.R., L.W.H. Leus, A.C. De Raedt, and E.J. Van Bockstaele. 2004. Occurrence of polyploidy in Rhododendron luteum Sweet, Hardy Ghent, and Rustica hybrids. The Azalean 26:32-37.
Eigsti, O.J. and P. Dustin. 1955. Colchicine in agriculture, medicine, biology, chemistry. Ames, Iowa: Iowa Univ. Press.
Eiselein, J.E. 1994. A study of chromosome yields and growth responses in colchicine treated rhododendrons. J. Am. Rhod. Soc. 48:205-209.
Francis, D. 1997. The stem cell concept applied to shoot meristems of higher plants. In: C.S. Potten (ed.). Stem cells. Academic Press, San Diego. 59-73.
Hancock, J.F. 1997. The colchicine story. HortScience 32:1011-1012.
Hosoda, T., A Moriya, and S. Sarahima. 1953. Chromosome numbers of satsuki, Rhododendron lateritium P1. Genetica 26:407-409.
Hudson, A. and J. Goodrich. 1997. Plant meristems: cell signaling keeps the balance. Current Biology 7:R427-R429.
Jones, J.R., T.G. Ranney, N.P. Lynch, and S.L. Krebs. 2007. Ploidy levels and genome sizes of diverse species, hybrids, and cultivars of Rhododendron . J. Am. Rhod. Soc. 61:220-227.
Kadota, M., and Y. Niimi. 2002. In vitro induction of tetraploid plants from diploid Japanese pear cultivar ( Pyrus pyrifolia N. cv. Hosui). Plant Cell Rep. 21:282-286.
Kehr, A.E. 1996a. Woody plant polyploidy. Am. Nurseryman 183:38-47.
Kehr, A.E. 1996b. Polyploids in rhododendron breeding. J. Am. Rhod. Soc. 50:215-217.
Kerstetter, R.A. and S. Hake. 1997. Shoot meristem formation in vegetative development. The Plant Cell 9:1001-1010.
Leach, D.G. 1961. Rhododendrons of the World and How to Grow Them. Charles Scribners' Sons, NY.
Lee, L.S. 1988. Citrus polyploidy—origins and potential for cultivar improvement. Aust. J. Agric. Res. 39:735-747.
Masterson, J. 1994. Stomatal size in fossil plants: evidence for polyploidy in majority of angiosperms. Science 264:421-423.
Morejohn, L.C., T.E. Bureau, J. Molè-Bajer, A.S. Bajer, and D.E. Fosket. 1987. Oryzalin, a dinitroaniline herbicide, binds to plant tubulin and inhibits microtubule polymerization in vitro. Planta 172:252-264.
Olsen, R.T., T.G. Ranney, and Z. Viloria. 2006. Reproductive behavior of induced allotetraploid xChitalpa and in vitro embryo culture of polyploid progeny. J. Amer. Soc. Hort. Sci. 131:716-724.
Paden, D.W. 1990. Doubling chromosomes with colchicines treatment in vitro as determined by chloroplast number in epidermal guard cells. J. Amer. Rhod. Soc. 44:162-165.
Pratt, C. 1983. Somatic selection and chimeras. In: J.N. Moore and J. Janick (eds.). Methods in Fruit Breeding. Purdue Unv. Press, West Lafayette, Ind. 172-185.
Pryor, R.L. and L.C. Frazier. 1968. Colchicine-induced tetraploid azaleas. HortScience 3:283-286.
Ramsey, J. and D.W. Schemske. 1998. Pathways, mechanisms, and rates of polyploidy formation in flowering plants. Annu. Rev. Ecol. Syst. 29:467-501.
Ranney, T.G. 2006. Polyploidy: From evolution to new plant development. Proc. Intern. Plant Propagators' Soc. 56:604-607.
Sanford, J.C. 1983. Ploidy manipulations. In: J.N. Moore and J. Janick (eds.). Methods in Fruit Breeding. Purdue Univ. Press, West Lafayette, Ind. 100-123.
Sakai, K., I. Miyajima, K. Ureshino, Y. Ozaki, and H. Okubo. 2004. Oryzalin-induced allotetraploids of an intersubgeneric hybrid between evergreen and deciduous azaleas. J. Fac. Agr. Kyushu Univ. 49: 293-299.
SAS Institute Inc. 1988. SAS/STAT User's Guide, Release 6.03 Edition. SAS Institute Inc., Cary, NC.
Semeniuk, P. and T. Arisumi. 1968. Colchicine-induced tetraploid and cytochimeral roses. Botanical Gazette 129:190-193.
Singh, R.J. 1993. Plant cytogenetics. CRC Press, Boca Raton, FL.
Soltis, D.E. and P.S. Soltis. 1993. Molecular data and the dynamic nature of polyploidy. Critical Rev. Plant Sci. 12:243-273.
Soltis, D.E., P.S. Soltis, and J.A. Tate. 2003. Advances in the study of polyploidy since Plant Speciation. New Phytol. 161:173-191.
Tax, F.E. and A. Durbank. 2006. Meristems in the movies: live imaging as a tool for decoding intercellular signaling in shoot apical meristems. The Plant Cell 18:1331-1337.
Tillney-Basset, R.A.E. 1986. Plant chimeras. Edward Arnold, London.
Tolstead, W.L. and J.F. Glencoe. 1991. Winter-hardy tetraploids of Rhododendron carolinianum and Rhododendron racemosum and their tetraploid hybrids. J. Am. Rhod. Soc. 45:83-84.
van Tuyl, J.M., B. Meijer, and M.P. van Diën. 1992. The use of oryzalin as an alternative for colchicine in in-vitro chromosome doubling of Lilium and Nerine . Acta Hort. 325:625-629.
Väinölä, A. 2000. Polyploidization and early screening of Rhododendron hybrids. Euphytica 112:239-244.
Wendel, J.F. 2000. Genome evolution in polyploids. Plant Mol. Bio. 42:225-249.
Widrlechner, M., H. Pellett, P. Ascher. 1982. Unreduced gametes in azalea hybrids: a possible breeding method for using promising azaleas of low fertility. J. Am. Rhod. Soc. 36:98-100.
Acknowledgments
Much appreciation is given to Mr. Joel Mowrey, Mr. Nathan Lynch, Dr. Darren Touchell, and the staff of the Mountain Horticultural Crops Research Station for their superb technical assistance. Partial funding for this project was provided by the Research Foundation of the American Rhododendron Society.
Authors
Jeff R. Jones is currently a Master of Science student, Thomas A. Eaker is a research specialist, and Thomas G. Ranney is a professor of Horticultural Science and member of the Southeastern Chapter.
* Name is not registered.
